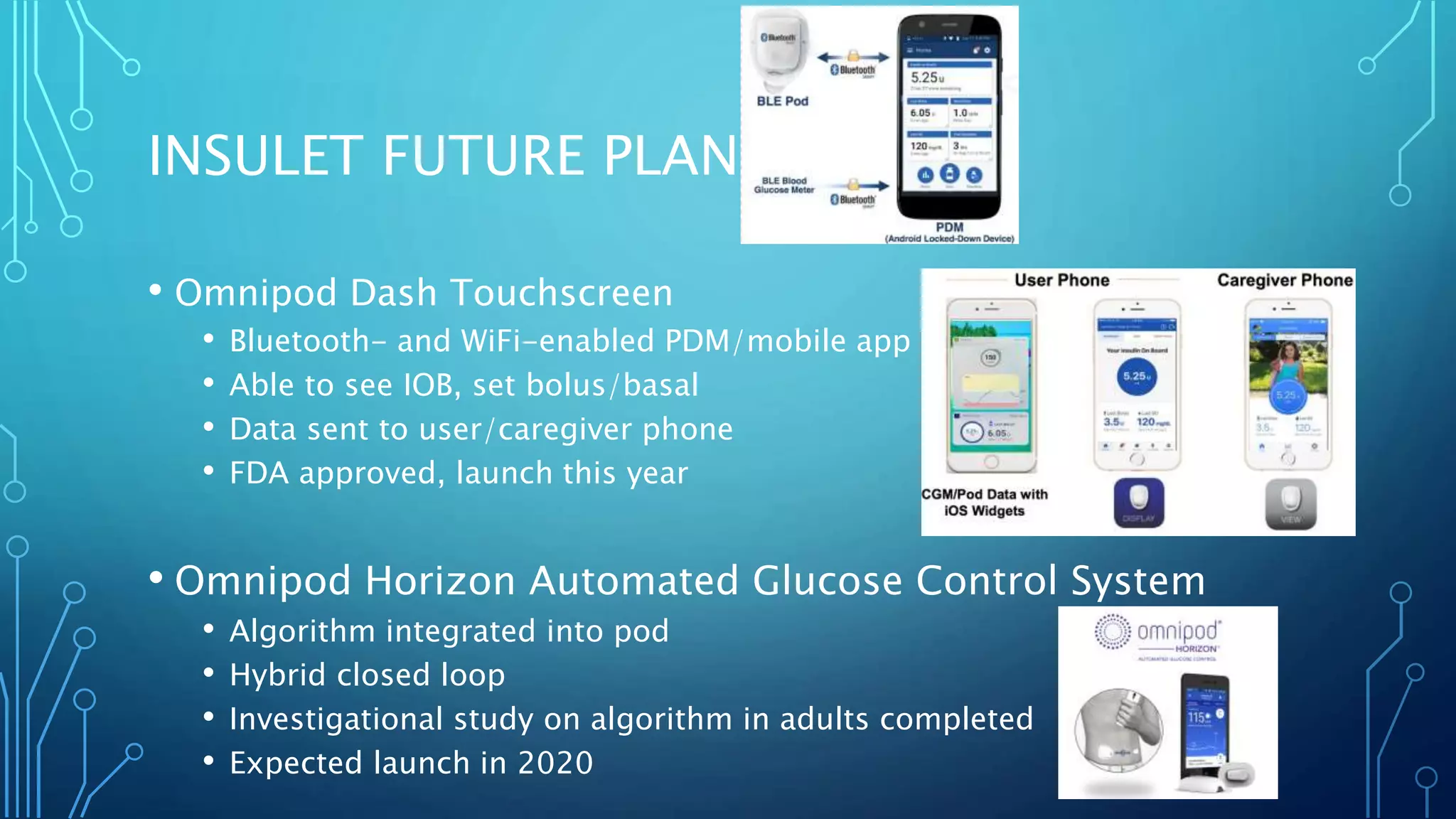
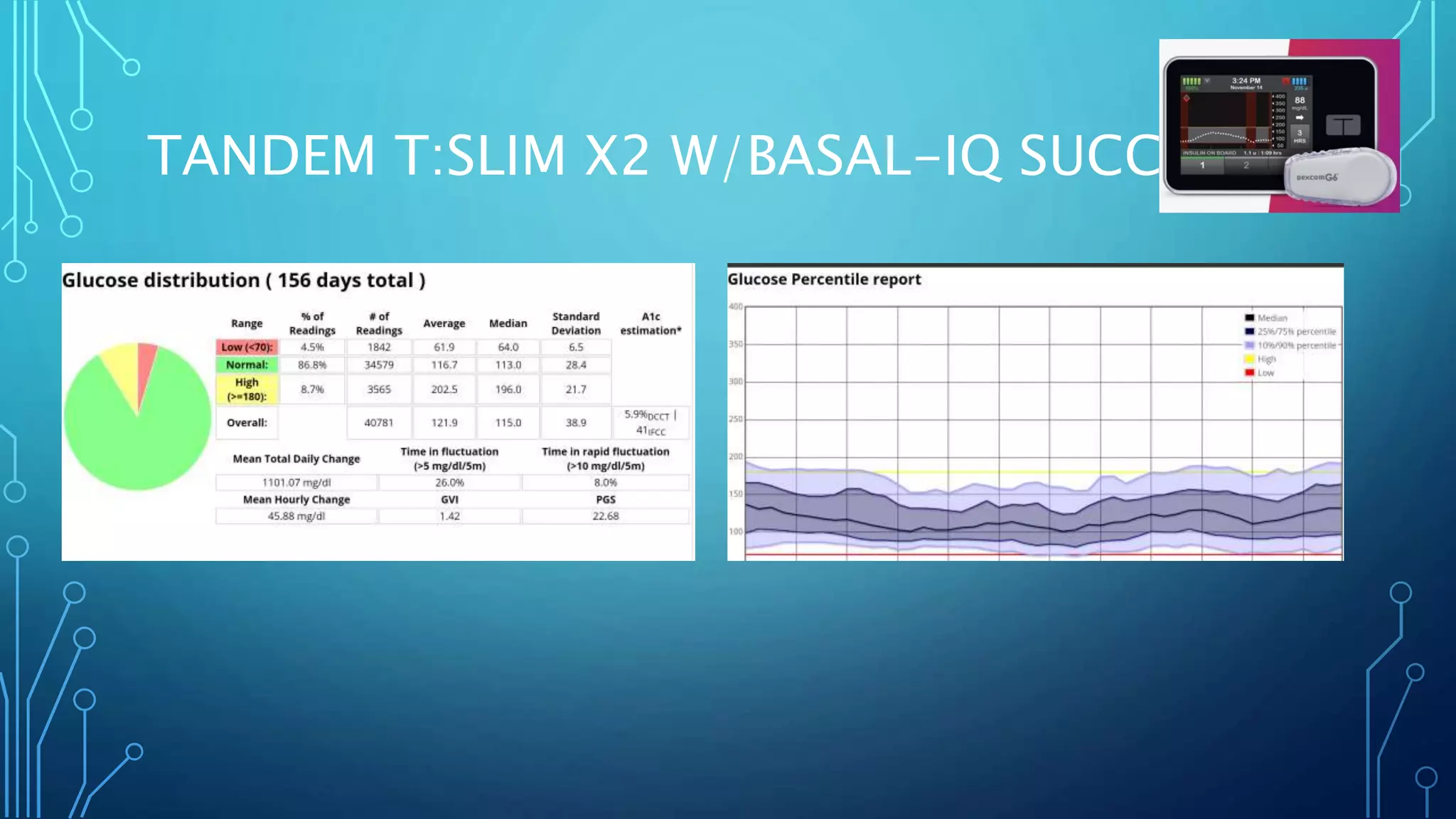
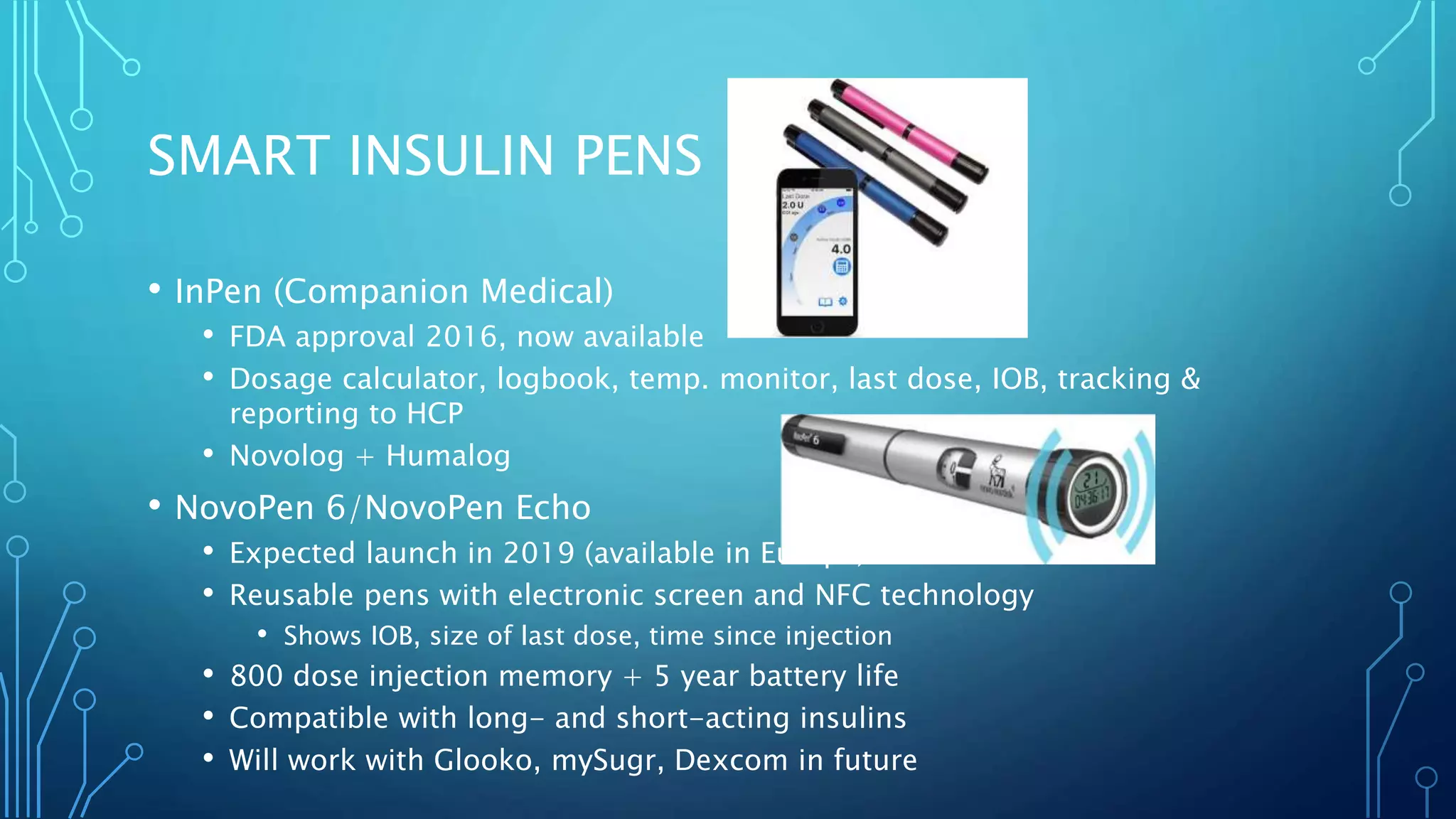
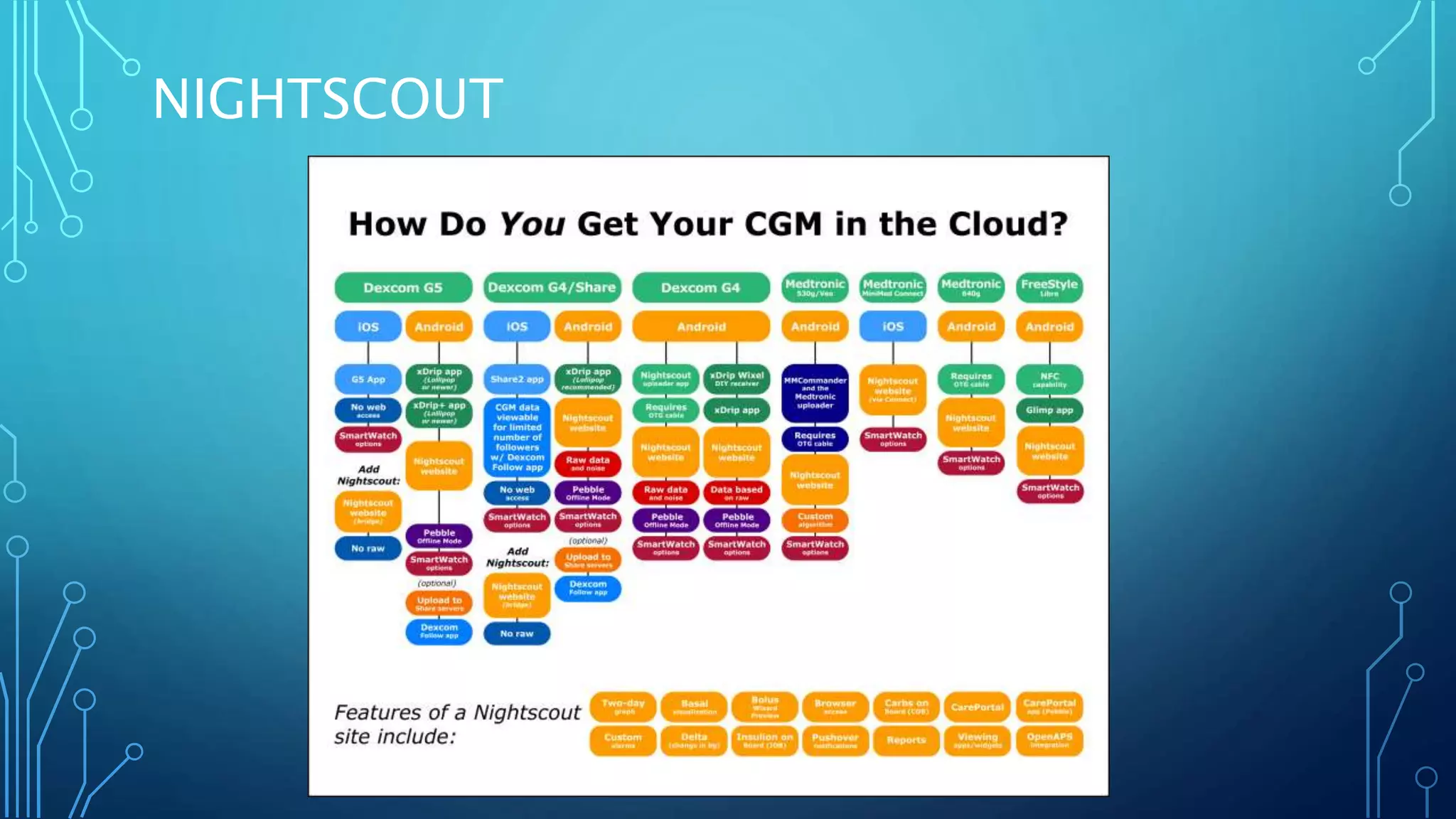
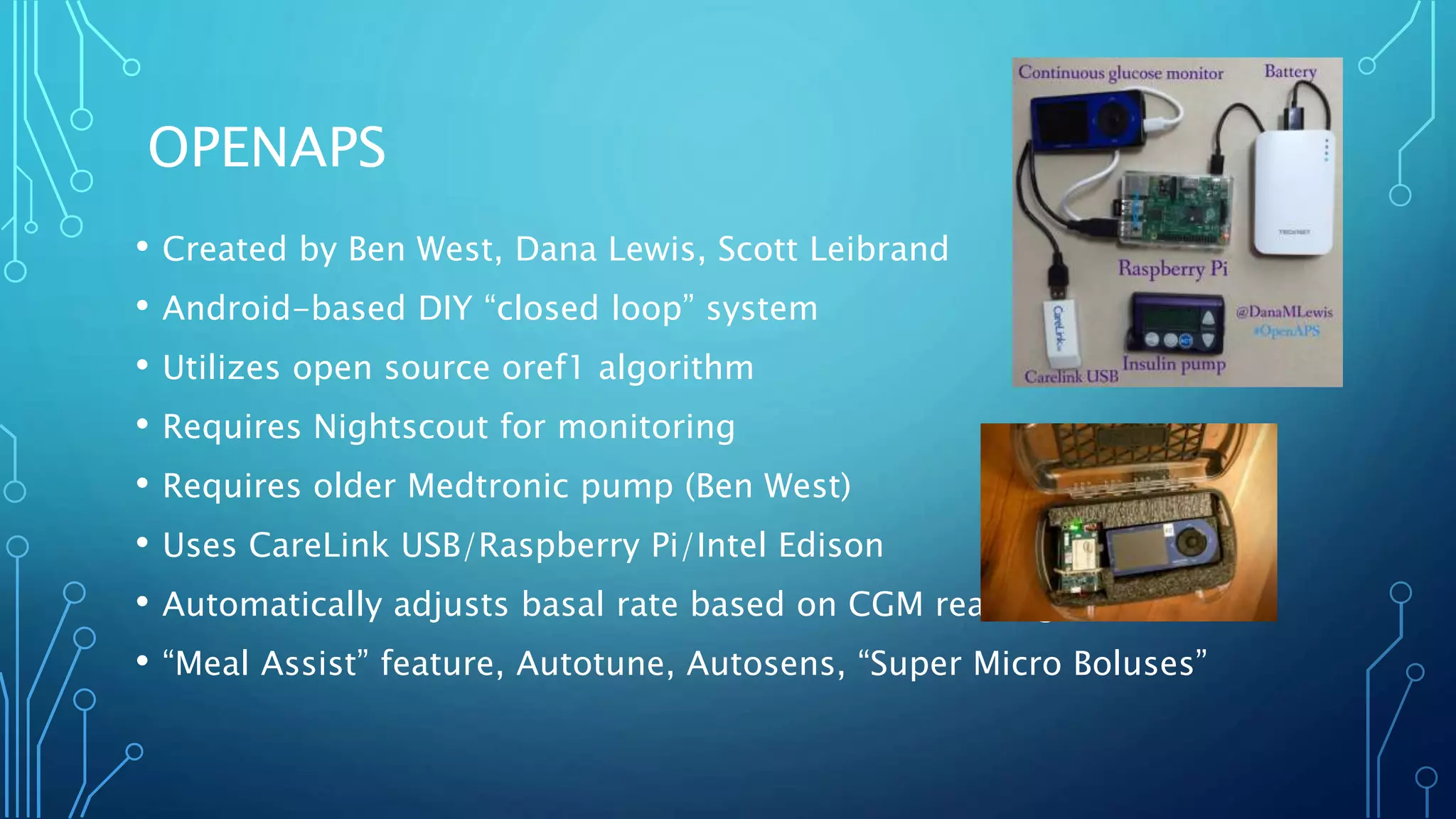
The document outlines various advancements in diabetes technology and management presented at the 2019 Type One Nation Summit in Tulsa, Oklahoma. It covers innovations such as insulin pumps, continuous glucose monitors, smart syringes, and mobile applications, along with future plans from companies like Medtronic, Tandem, and Dexcom. The discussion emphasizes the integration of technology for better diabetes management and introduces DIY systems and upcoming products designed to improve patient care.