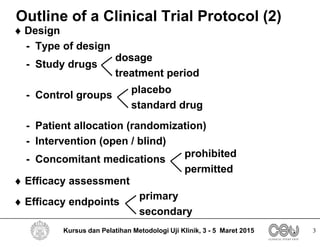
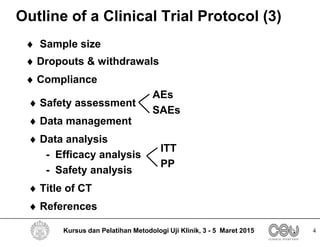
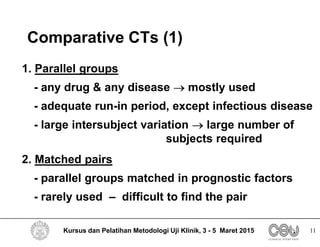
This document outlines the key elements that should be included in a clinical trial protocol. It discusses including an introduction describing the disease, drug, study rationale and objectives. It also describes the importance of defining the study population through inclusion and exclusion criteria. The protocol should specify the study design, including type of design, study drugs, control groups, randomization, blinding and treatment duration. It also notes key aspects like efficacy and safety assessments, sample size calculation, compliance monitoring and data analysis plans. The protocol provides guidance on the level of detail needed to properly plan and regulate a clinical trial.