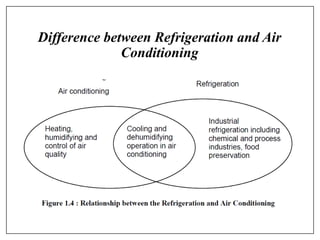
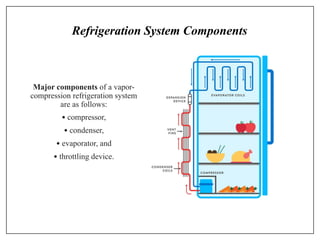
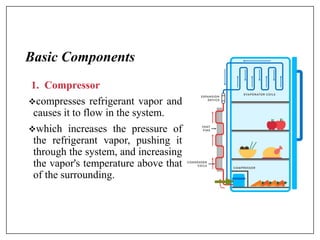
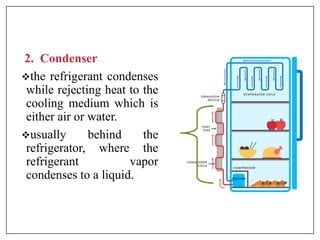
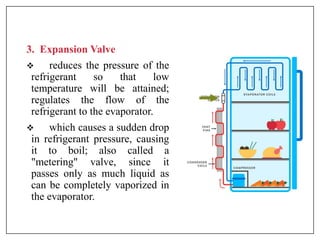
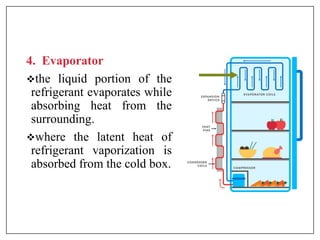
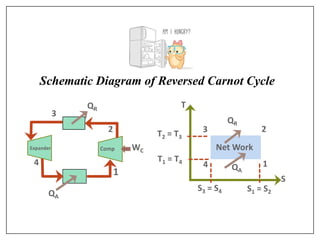
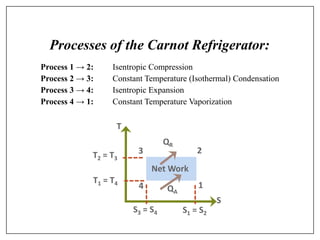
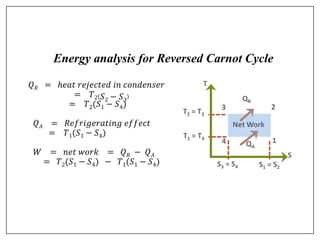
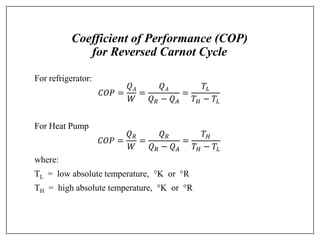
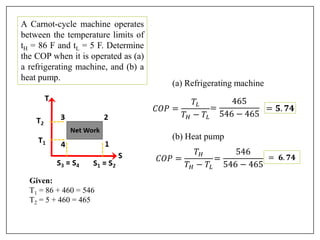
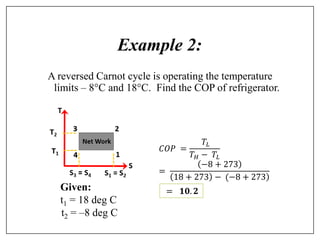
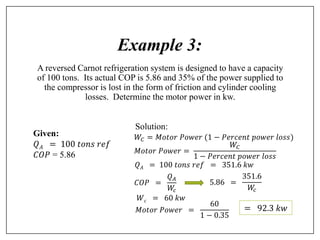
This document provides an introduction to refrigeration systems, explaining the differences between refrigeration and air conditioning, essential components, and their historical development. It covers key concepts such as tons of refrigeration, the reversed Carnot cycle, and the coefficient of performance (COP). Additionally, it discusses the practical applications of refrigeration systems and includes exercises for further learning.