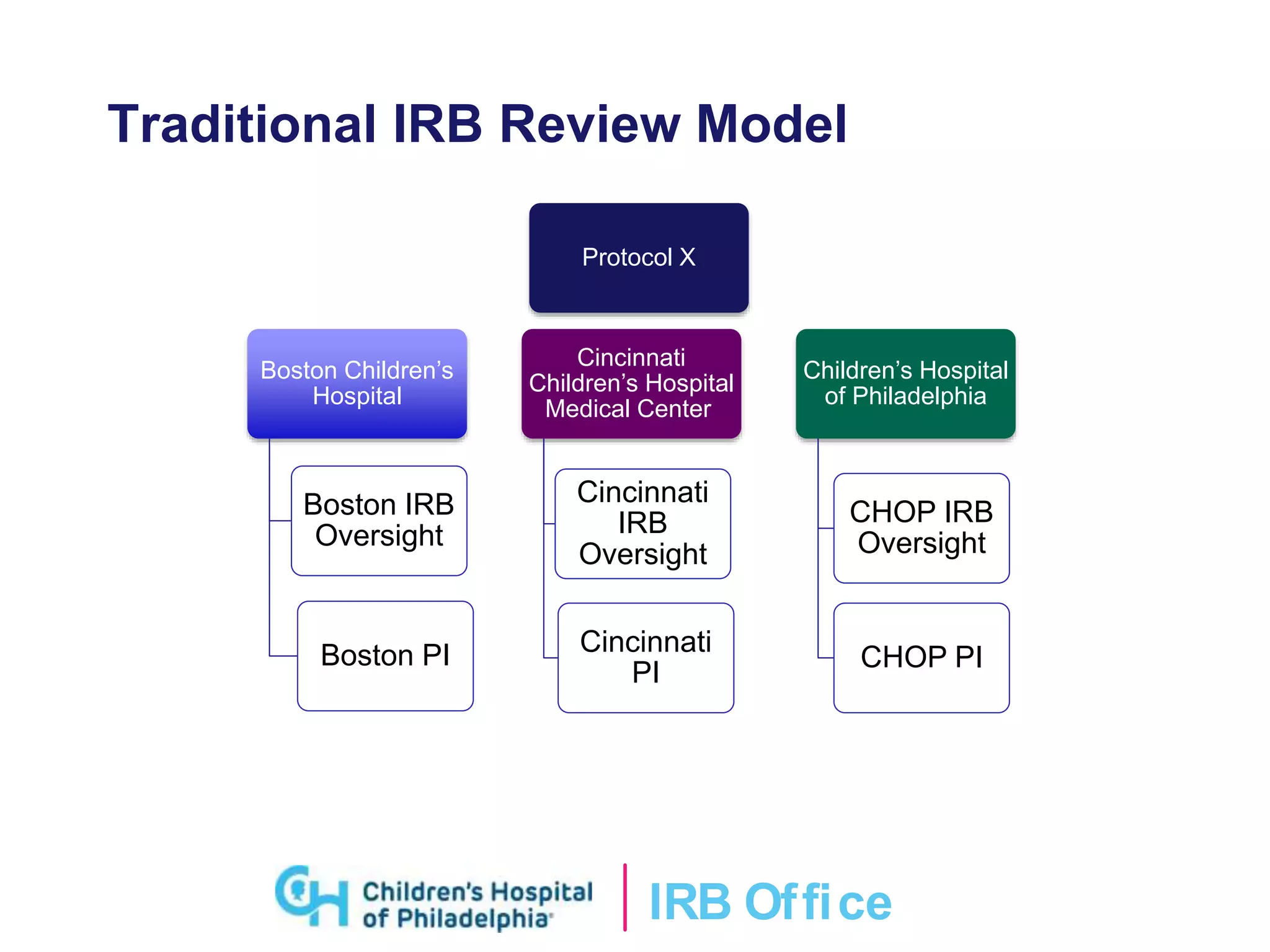
This document provides an overview of the single IRB review requirement and CHOP's processes for serving as both a reviewing IRB and relying institution. It defines key terminology, describes the responsibilities of reviewing and relying institutions, and outlines CHOP's procedures for submitting protocols and amendments when acting as the reviewing IRB or relying on an external IRB. Frequently asked questions are also addressed. Upcoming changes to the eIRB system aim to streamline review of multi-site studies when CHOP serves as the reviewing IRB.