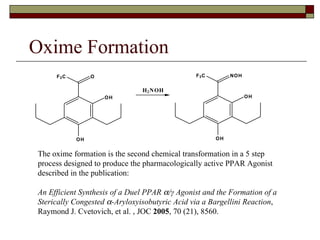
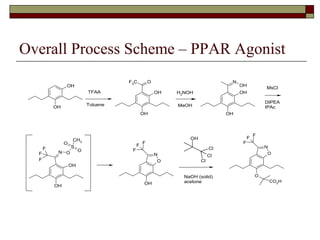
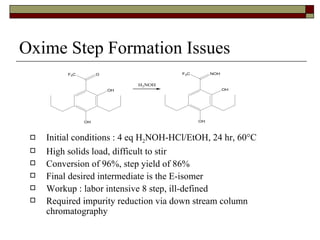
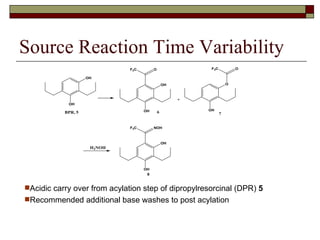
The document summarizes optimization work done on the oxime formation step in a multi-step process to produce a PPAR agonist drug. Initial issues with the step included high solids loading, long reaction time, and need for an extensive purification. Screening reaction conditions identified hydroxylamine free base and methanol as a solvent improved the reaction time and yield. Further, crystallizing the oxime product as a salt eliminated the need for an extractive workup, simplifying purification. The optimized process resulted in over 90% yield in under 18 hours with a clean crystalline product.