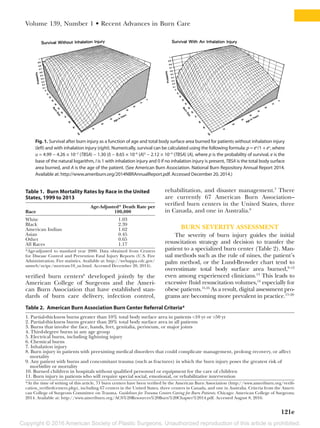
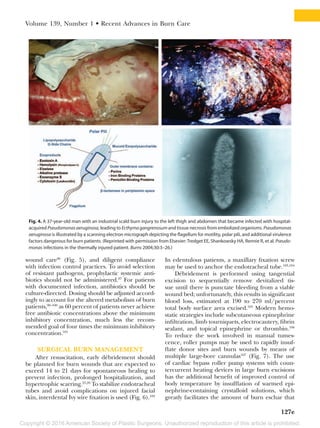
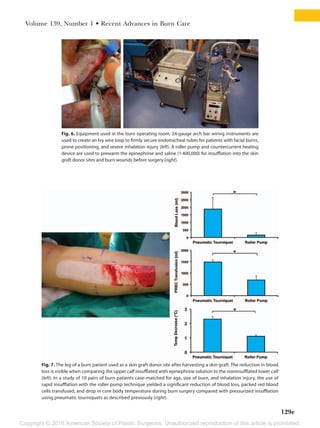
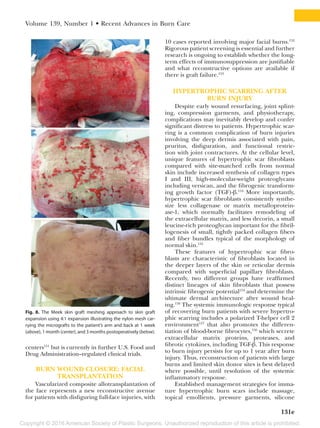
This document summarizes recent developments in burn care management. It describes the epidemiology of severe burns, noting factors such as gender, age, cause and mortality rates varying by factors like race. It also discusses challenges in assessing burn depth and severity, and the development of new tools like laser Doppler imaging to improve accuracy. The document outlines recent advances in areas like fluid resuscitation, wound management, sepsis prevention and reconstruction that have improved outcomes, but also new risks requiring adjusted care like infections and thromboembolic events. The overall evolution of burn care aims to further enhance survival and quality of life for burn patients.