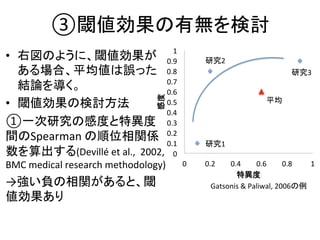
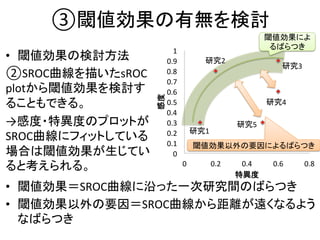
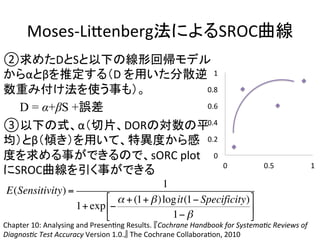
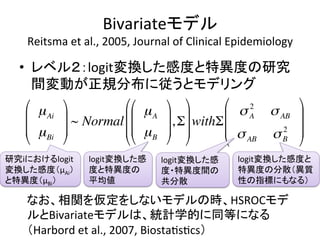
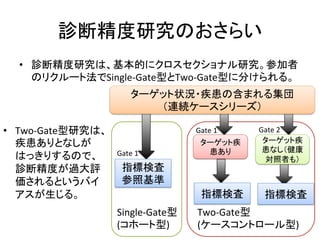
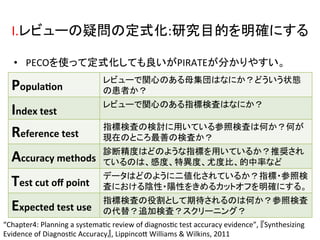
1. The document summarizes the planning of a systematic review to evaluate the diagnostic accuracy of tests. It outlines the key components of planning a diagnostic test accuracy review, including defining the population, index test, reference standard, and accuracy measures to be evaluated.
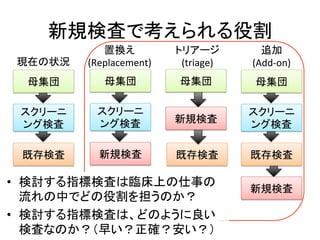
2. It describes the two main types of diagnostic reviews: single-gate and twin-gate designs. Key elements that need to be addressed are the inclusion criteria, assessment of risk of bias, analysis of heterogeneity.
3. The expected use of the index test and threshold or cut-off values used to define positive or negative results are also important planning considerations.














![Zhu[29], 2012, China 159 (71%) 42 (18,62) unclear METAVIR $15 mm unclear
Ucar[18], 2013, Turkey 73 (64%) 42.81612.86 unclear METAVIR unclear Yes
Gong[21], 2013, China 41 (73%) 50.8610.3 unclear METAVIR unclear unclear
Wang[20], 2013, China 231 (68%) 34.169.8 ,1d Scheuer .15 mm Yes
Ji[17], 2011, China 313 (69%) 35.6611.2 1d METAVIR 20 mm unclear
Bas¸ar[25], 2013, Turkey 76 (55%) unclear ,1d METAVIR .10 mm Yes
Bonnard[19], 2010, France 59 (68%) 3569 0.5–10 m METAVIR 2166 mm Yes
Erdogan[11], 2013, Turkey 221 (63%) 43.68612.56 #1d Ishak unclear Yes
Wu[30], 2010, China 78 (85%) 32.6612.3 unclear METAVIR .15 mm unclear
Mallet[16], 2009, France 138 (71%) 42615 ,1d METAVIR 17.666.8 unclear
Seto[24], 2011, China 237 (68%) 38.2 (18,63) same time Ishak $15 mm Yes
Zhu[27], 2011, China 175 (78%) 36.569.4 #7d METAVIR .15 mm Yes
Liu[23], 2012, China 114 (80%) 38.32611.36 same time METAVIR 15,20 mm unclear
Wang[26], 2013, China 149 (93%) 37 (30,42) #2d Scheuer .10 mm Yes
Xun[28], 2013, China 197 (76%) 31 (21–45) same time Scheuer .15 mm unclear
Zhang[32], 2009, China 86 (60%) 39 (16–64) ,1d METAVIR 15,20 mm unclear
Zhang[22], 2012, China 361 (62%) 36611 #7d Scheuer unclear unclear
Zhang[31], 2010, China 212 (88%) 3167 1day Scheuer 20 mm Yes
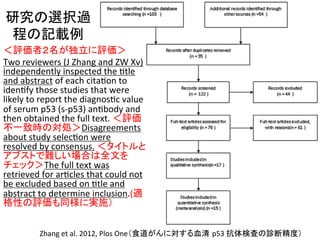
グ㍕䠖QUADS-‐2䜢⏝䛔䛯୍ḟ◊✲䛾㉁
䛾ホ౯䠄䝥䝻䝖䝁䝹䛸ㄽᩥグ㍕䠅
䝥䝻䝖䝁䝹䛷䛾㉁䛾ホ౯䛻㛵䛩䜛グ㍕䠖Two
review
authors
(JFC,
MC)
will
independently
assess
the
methodological
quality
of
each
study
using
a
four
domain
tool
adapted
from
QUADAS-‐2
(WhiQng
2011a).
ኚ᭦⟠ᡤ䜒ㄝ᫂We
tailored
the
quality
assessment
tool
to
our
review
quesQon.
Cohen
et
al.,
(2013)
Cochrane
Protocol䚷
䠄Ꮚ䛹䜒䛾ဗ㢌⅖䛻䛚䛡䜛A⩌䝺䞁䝃⌫
Literature and search strategy
QUADAS-‐2䛾せ⣙䛾グ㍕䠚
⳦䜢᳨ฟ䛩䜛ᛴ㏿ᢠయ᳨ᰝ䛾⢭ᗘ䠅
←⾲䛷せ⣙
Mohamed
et
al.,
CMAJ,
2014
ᅗ䛷せ⣙→
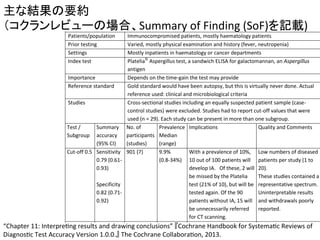
Yuanyuan
et
al.,
Plos
one,
2014
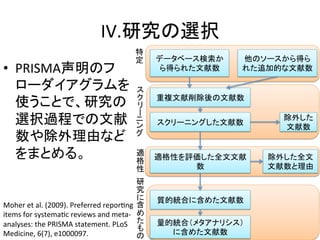
The following databases were searched without the use of time
limitations: PubMed, Ovid, EMBASE, the Cochrane Library, the
Chinese National Knowledge Infrastructure (CNKI) and the
Chinese Biology Medicine disc (CBMdisc). The search strategy to
identify all relevant articles involved the use of the following key
words: FIB-4, aspartate aminotransferase, AST, alanine amino-transferase,
ALT, platelet, PLT, hepatitis B, fibrosis and cirrhosis.
For example, File S1 and S2 displayed the search strategy of Ovid
and PubMed respectively. Additional studies were identified via a
manual review of the reference lists of identified studies and review
articles. This literature search was performed in November 2013.
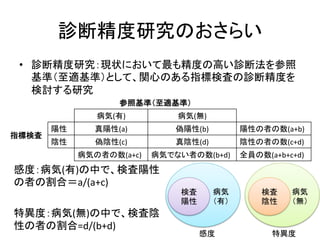
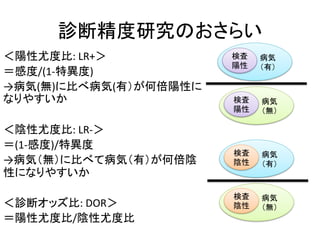
Inclusion criteria
Studies were deemed eligible if they met the following inclusion
criteria: 1) the study evaluated the performance of the FIB-4 index
for the diagnosis of fibrosis in mono-HBV-infected patients before
antiviral therapy. Studies including patients with other causes of
liver disease were included if data of HBV-infected patients could
be extracted. 2) Liver biopsy was used as the reference standard for
assessing fibrosis. METAVIR [8] or comparable staging systems
doi:10.1371/journal.pone.0105728.t001](https://image.slidesharecdn.com/random-141025091252-conversion-gate01/85/slide-22-320.jpg)