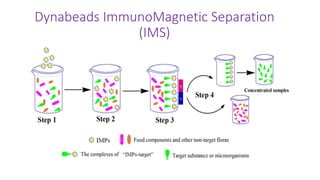
This document provides instructions and information for Lab #3 on antigen-antibody interactions focusing on Salmonella detection. Dynabeads coated with antibodies specific for Salmonella are used to isolate the bacteria from pre-enriched samples via immunomagnetic separation. The bead-bacteria complexes are then grown on selective media to confirm the presence of Salmonella. Key steps include incubating the sample with Dynabeads, separating complexes using a magnetic particle concentrator, washing, and plating on HEK and XLT-4 agar. The document outlines the target microorganism Salmonella enterica, characteristics of selective and differential media, and important techniques for efficient immunomagnetic separation.