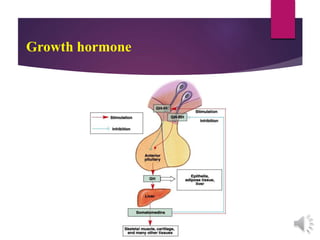
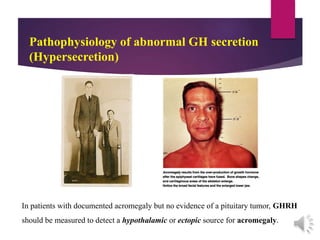
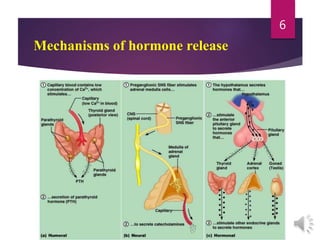
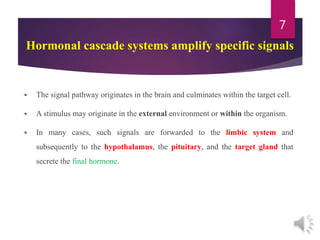
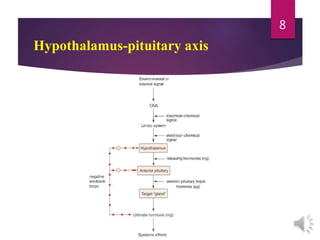
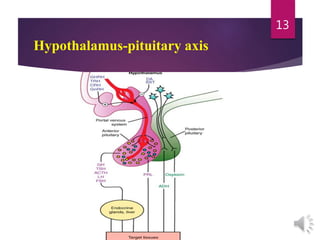
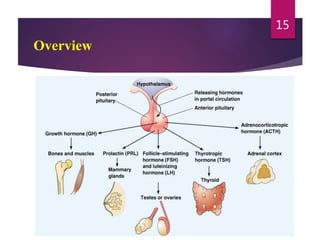
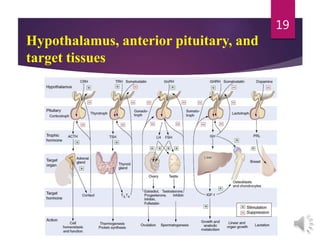
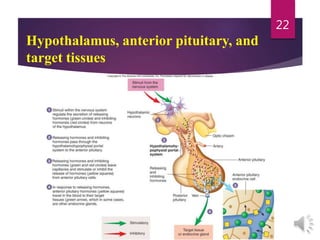
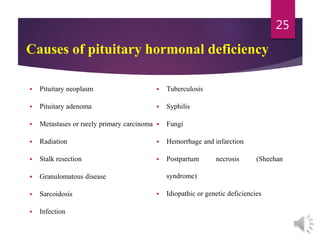
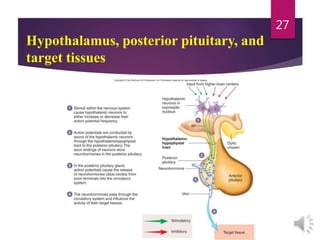
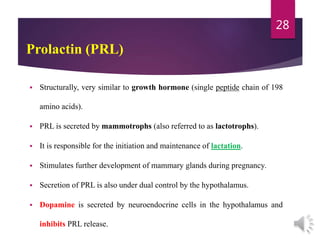
The document discusses the hypothalamus-pituitary axis. It describes how the hypothalamus secretes releasing hormones that stimulate the anterior pituitary to release hormones like growth hormone, prolactin, and thyroid-stimulating hormone. The pituitary hormones then target various endocrine glands like the thyroid to regulate processes like metabolism and development. Disruptions to the hypothalamus-pituitary communication can lead to hormonal deficiencies. The feedback loops between the hypothalamus, pituitary and target glands allow for coordinated control of the endocrine system.


















![Hyperprolactinemia
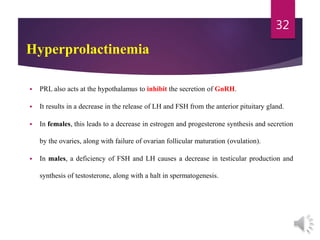
Another important cause of hyperprolactinemia is hypothyroidism.
TRH stimulates PRL secretion, thus explaining the mild hyperprolactinemia seen in both
primary (thyroid) and secondary (pituitary) hypothyroidism.
Thyroid function tests (free thyroxine [FT4] and TSH) are always indicated to rule out
hypothyroidism when a patient with hyperprolactinemia is evaluated.
33](https://image.slidesharecdn.com/hormone2-220905071142-9882a492/85/Hormone-2-pptx-33-320.jpg)