polymer .pptx
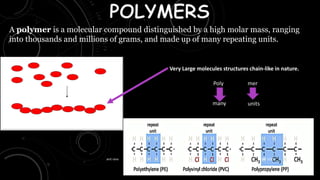
- 1. A polymer is a molecular compound distinguished by a high molar mass, ranging into thousands and millions of grams, and made up of many repeating units. Very Large molecules structures chain-like in nature. Poly mer many units 22-07-2023 anit rana 1
- 2. •The process by which the monomer molecules are linked to form a big polymer molecule is called ‘polymerization’. •Polymerization is a process of bonding monomer, or “single units” together through a variety of reaction mechanisms to form longer chains named Polymer Polymerization 22-07-2023 anit rana 2
- 4. BASED ON ORIGIN OF SOURCE Natural Polymer :- Polymers which are isolated from natural materials are called as Natural Polymers . E.g. : Cotton , silk , wool , rubber . Synthetic Polymer :- Polymers which are synthesized from low molecular weight compounds are called as Synthetic Polymers . E.g. : Polyethylene , nylon , terylene. Semisynthetic Polymers :- These polymers are mostly derived from naturally occurring polymers by chemical modification . E.g. : Rayon 22-07-2023 anit rana 4
- 5. BASED ON STRUCTURE Linear Polymer : Molecules form long chains without branches. They can be place one above the other and are closely packed in space. Results in high density, tensile strength and high M.P . Eg: HDP, PVC Branched polymers Their molecules are irregularly packed hence they have low density, Tensile strength and melting point. E.g. LDPE. They have straight long chain with different side chain. Cross-Linked Polymer : The links involved are called cross links. They are hard, rigid .and brittle due to their network structure. E.g. Bakelite, Melamine, Formaldehyde resins, Vulcanized rubber It includes interconnections between chains . 22-07-2023 anit rana 5
- 6. BASED ON MODE OF POLYMERISATION Addition Polymerization Condensation Polymerization Monomers must have either a double bond or triple bond Monomers must have two similar or different functional groups Produces no by-products By-products such as ammonia, water and HCl are produced Addition of monomers results in polymers. Initiator are needed Condensation of monomers result in polymers. No initiators. The molecular weight of the resultant polymers is a multiple of monomer’s molecular weight The molecular weight of the resultant polymer is not a multiple of monomer’s molecular weight Lewis acids or bases, radical initiators are catalysts in addition polymerization Catalyst used in condensation are acids, base, metal compounds etc. Common eg of addition polymerization are PVC, polyethene, Teflon etc. Common examples of condensation polymerization are nylon, bakelite, etc. 22-07-2023 anit rana 6
- 10. Based on molecular forces Thermoplastics: The relatively weak intermolecular forces hold the molecules in a thermoplastic together, so that the material softens when exposed to heat and then returns when cooled to its original condition. Thermoplastics are the bulk of linear and slightly branched polymers. Chain polymerization produces all the major thermoplastics. Food packaging, insulation, vehicle bumpers and credit cards are some examples. Thermosets: Heating cannot reshape the thermosets. Thermosets are usually three-dimensional networked polymers in which polymer chains have a high degree of cross-linking. The cross linking limits the chains ' movement and contributes to a solid material that makes thermosets strong and robust. They are predominantly used in the automotive and construction industries. They are also used for making toys, varnishes, hulls of boats and glues 22-07-2023 anit rana 10
- 11. Elastomers: Elastomers are rubbery polymers that can be extended easily to their unstretched length many times and that quickly return to their original dimensions when the pressure is released. Elastomers are cross- linked but have a low density of cross-link. Elastomers are composed from rubber bands and other elastics. If polymer is drawn into long filament like materiel whose length is at least 100 times it’s diameter, are said to be converted into fiber. They have high tensile strength because of high intermolecular attractive force like Hydrogen bonding. Highly crystalline. E.g. Nylon, Terylene. Fibre 22-07-2023 anit rana 11
- 12. Low density polythene (LDPE) •Low density polythene is prepared by a number of methods. The common method for the preparation of LDPE is polymerization of ethylene monomer at high pressure (1000 - 2000 atm) and temperature (2500C) in presence of oxygen peroxide hydro peroxide( free radical generator). In this process huge branched chains are formed through out every long back bone chain. The presence of branches repel each other and the long chains are not well fitted to each other having some gap produce low density (0.91 to 0.925 gm/cc) polymer. Preparation of some important addition polymers Characteristics of low density polythene: a) Low density polyethylene have density is low (0.91 to 0.925 gm/cc). b) It is branched chain addition polymer. c) Semi crystalline polymer having crystallinity 45-50 percent. d) Chemically inert, nonpolar and having dielectric property zero. e) Tough but flexible. Uses of low density polythene: a) As LDPE is a good insulator they normally used for the preparation of electrical wires and cables. b) Pouch pack, squeeze bottles, delivery pipes are prepared from LDPE.c) Toys, refill for ball pen and ball pen also prepared from LDPE. 22-07-2023 anit rana 12
- 13. High density polythene can be prepared by the polymerization of ethylene monomer at low pressure (5 - 7 atm.) and temperature (60−700C) in presence of Zieglar Natta catalyst like triethyl aluminium and titanium tetrachloride. In this process of polymerization as there is absence of branch through out the long chain back bone, the chains are well fitted to each other which makes the polymer a high density (0.95 to 0.97 gm/cc) polymer. High density Polythene(HDPE) Characteristics of high density polythene: a) High density polyethylene have high density (0.95 to 0.97 gm/cc). b) It is less branched. c) Highly crystalline polymer having crystallinity 80-90 percent. d) Chemically inert, nonpolar and having dielectric property zero. e) Highly tough but flexible. Uses of high density polythene: a) As HDPE is a good insulator they normally used for the preparation of high performance electrical cables. b) Due to inertness it is used for the storage of H2SO4, pipes for LPG gas and water reserver also. 22-07-2023 anit rana 13
- 14. Polytetrafluoroethene (Teflon/PTFE) Teflon is manufactured by heating tetrafluoroethene with a free radical or persulphate catalyst at high pressures. It is chemically inert and resistant to attack by corrosive reagents. It is used in making oil seals and gaskets and also used for non – stick surface coated utensils. Polyacrylonitrile/PAN The addition polymerisation of acrylonitrile in presence of a peroxide catalyst leads to the formation of polyacrylonitrile. Polyacrylonitrile is used as a substitute for wool in making commercial fibres as Orlon. It is used for making synthetic carpets. 22-07-2023 anit rana 14
- 15. TERYLENE OR DACRON Polyamides Nylon 6,6: It is prepared by the condensation polymerisation of hexamethylenediamine with adipic acid under high pressure and at high temperature. Polyesters are used in fibers, films, and plastic bottles. Nylon 6, 6 is used in making sheets, bristles for brushes and in textile industry. 22-07-2023 anit rana 15
- 16. Nylon 6: It is obtained by heating caprolactum with water at a high temperature. Nylon 6 (also called Perlon) is used for making strong, flexible fibers for ropes and tire cord. 22-07-2023 anit rana 16
- 17. Phenol - formaldehyde polymer Bakelite and related polymers) Bakelite is a polymer made up of the monomers phenol and formaldehyde. This phenol-formaldehyde resin is a thermosetting polymer These are obtained by the condensation reaction of phenol with formaldehyde in the presence of either an acid or a base catalyst. The reaction starts with the initial formation of o-and/or p- hydroxymethylphenol derivatives, which further react with phenol to form compounds having rings joined to each other through –CH2 groups. The initial product could be a linear product – Novolac used in paints 22-07-2023 anit rana 17
- 18. Novolac on heating with formaldehyde undergoes cross linking to form an infusible solid mass called bakelite. It is used for making combs, phonograph records, electrical switches and handles of various utensils. 22-07-2023 anit rana 18
- 19. Melamine – formaldehyde polymer Melamine formaldehyde polymer is formed by the condensation polymerisation of melamine and formaldehyde. It is used in the manufacture of unbreakable crockery. 22-07-2023 anit rana 19
- 20. Copolymerisation A copolymer is a polymer that is made up of two or more monomer species. Copolymers have properties quite different from homopolymers. For example, butadiene - styrene copolymer is quite tough and is a good substitute for natural rubber. It is used for the manufacture of autotyres, floortiles, footwear components, cable insulation, etc. 22-07-2023 anit rana 20
- 21. Natural rubber Rubber is probably the best known organic polymer and the only true hydrocar- bon polymer found in nature. Natural rubber is poly-cis-isoprene, which is extracted from the tree which is a colloidal dispersion of rubber in water. It is formed by the radical addition of the monomer isoprene. Actually, polymerization can result in either poly-cis-isoprene or poly-trans- isoprene—or a mixture of both, depending on reaction conditions: The cis-polyisoprene molecule consists of various chains held together by weak van der Waals interactions and has a coiled structure. Thus, it can be stretched like a spring and exhibits elastic properties. 22-07-2023 anit rana 21
- 22. Vulcanisation of rubber: Vulcanized rubber has much greater toughness and elasticity than natural rubber. It withstands relatively high temperatures without softening, and it remains elastic and flexible when cold. Natural rubber becomes soft at high temperature (>335 K) and brittle at low temperatures (<283 K) and shows high water absorption capacity. It is soluble in non-polar solvents and is non-resistant to attack by oxidising agents. On a molecular level, vulcanization causes cross-linking of the cis-1,4- polyisoprene chains through disulfide - S - S - bonds, similar to the cystine bridges that link peptides . In vulcanized rubber, the polymer chains are linked together so they can no longer slip past each other. When the material is stressed, the chains stretch, but cross-linking prevents tearing. 22-07-2023 anit rana 22
- 23. In the manufacture of tyre rubber, 5% of sulphur is used as a crosslinking agent. The probable structures of vulcanised rubber molecules are depicted below 22-07-2023 anit rana 23
- 24. Preparation of Synthetic Rubbers Neoprene or polychloroprene is formed by the free radical polymerisation of chloroprene. It has superior resistance to vegetable and mineral oils. It is used for manufacturing conveyor belts, gaskets and hoses. 22-07-2023 anit rana 24
- 25. Buna – N Buna –N is obtained by the copolymerisation of 1, 3 – butadiene and acrylonitrile in the presence of a peroxide catalyst. It is resistant to the action of petrol, lubricating oil and organic solvents. It is used in making oil seals, tank lining, etc. 22-07-2023 anit rana 25
- 26. Biodegradable Polymers Poly β-hydroxybutyrate – co-β-hydroxy valerate (PHBV) PHBV is used in speciality packaging, orthopaedic devices and in controlled release of drugs. PHBV undergoes bacterial degradation in the environment. 22-07-2023 anit rana 26
- 27. Nylon 2–nylon 6 It is an alternating polyamide copolymer of glycine (H N–CH –COOH) 22 and amino caproic acid [H2N (CH2)5 COOH] and is biodegradable. 22-07-2023 anit rana 27
- 28. Some Other Commercially Important Polymers 22-07-2023 anit rana 28