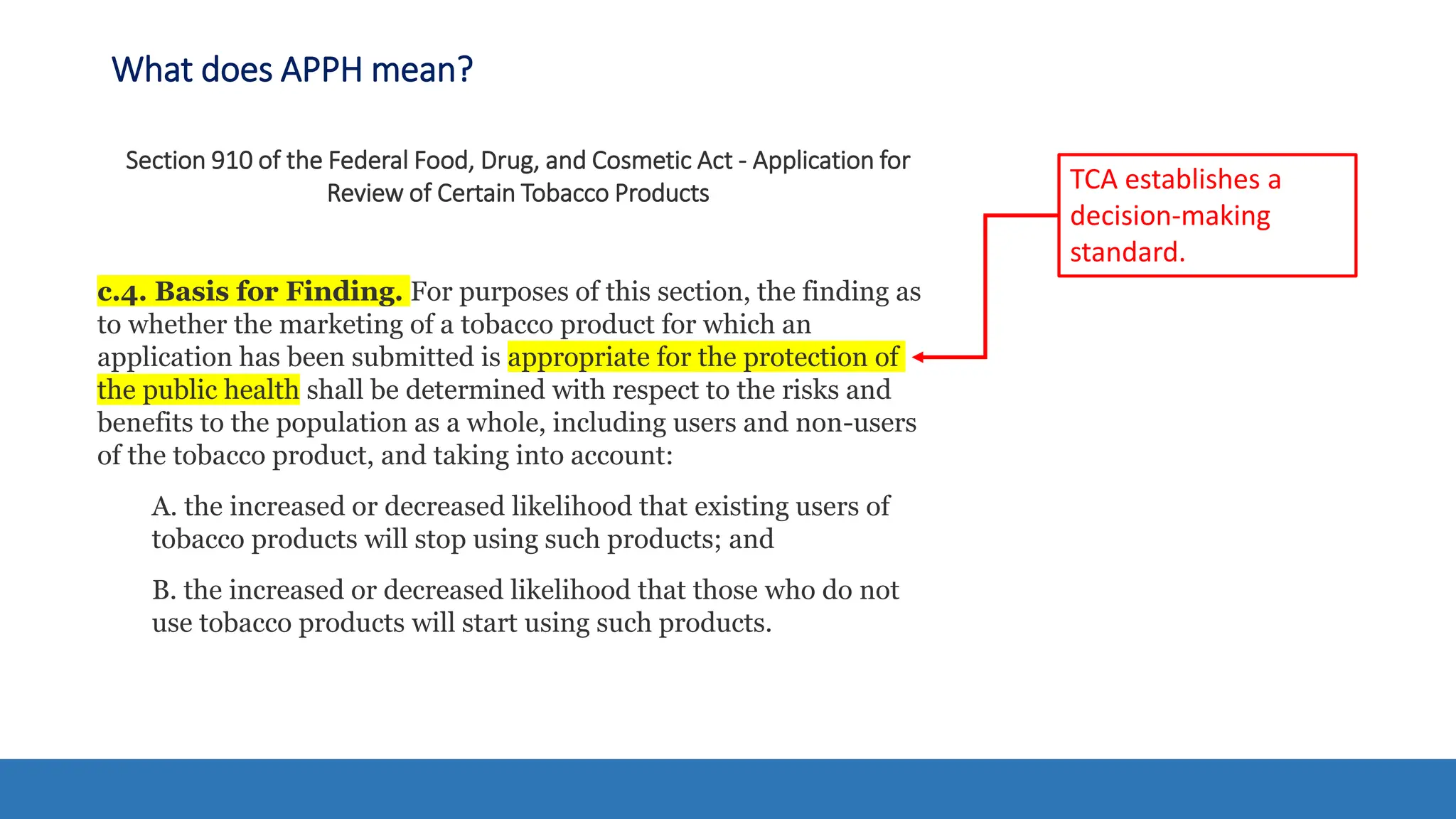
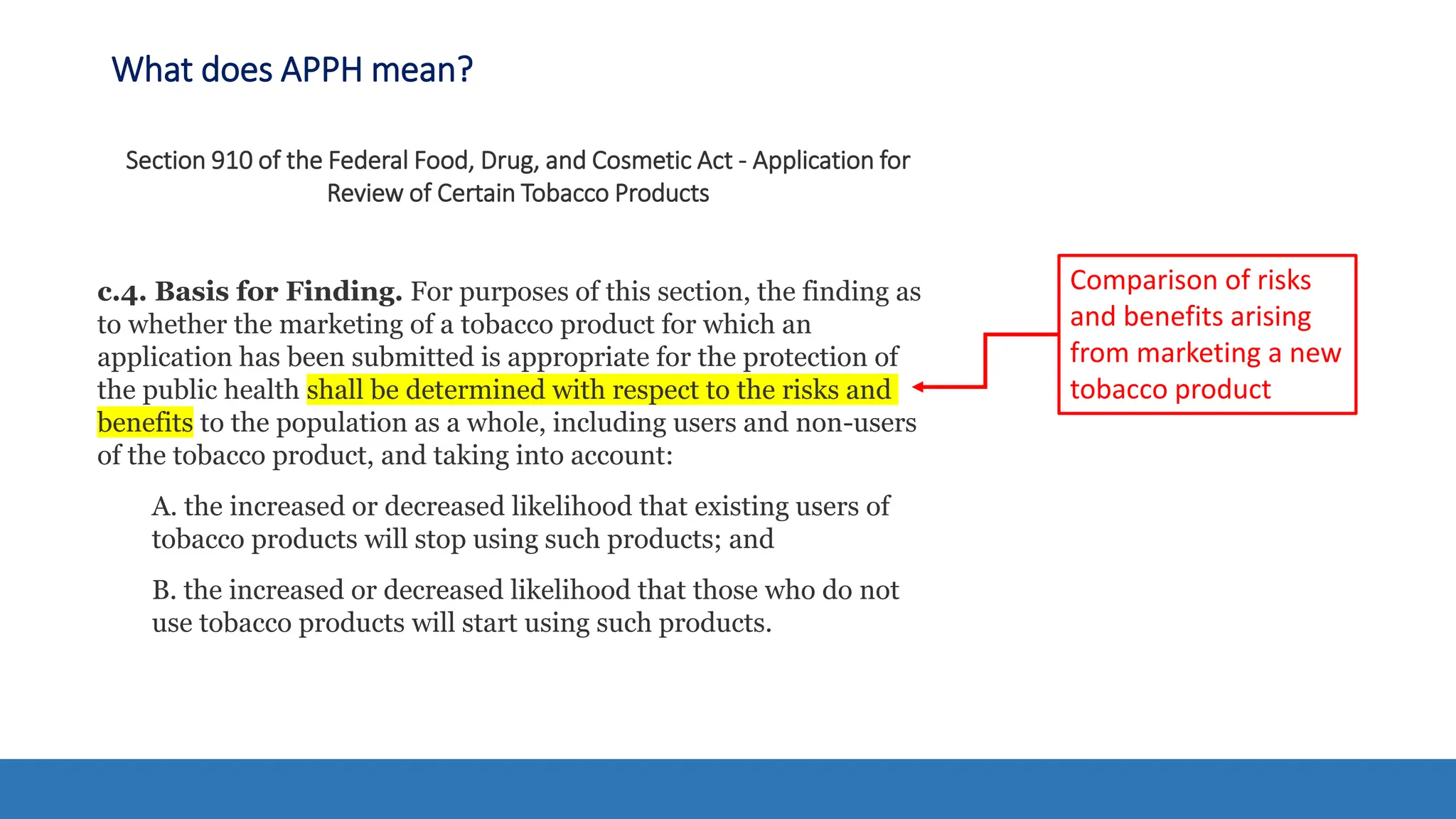
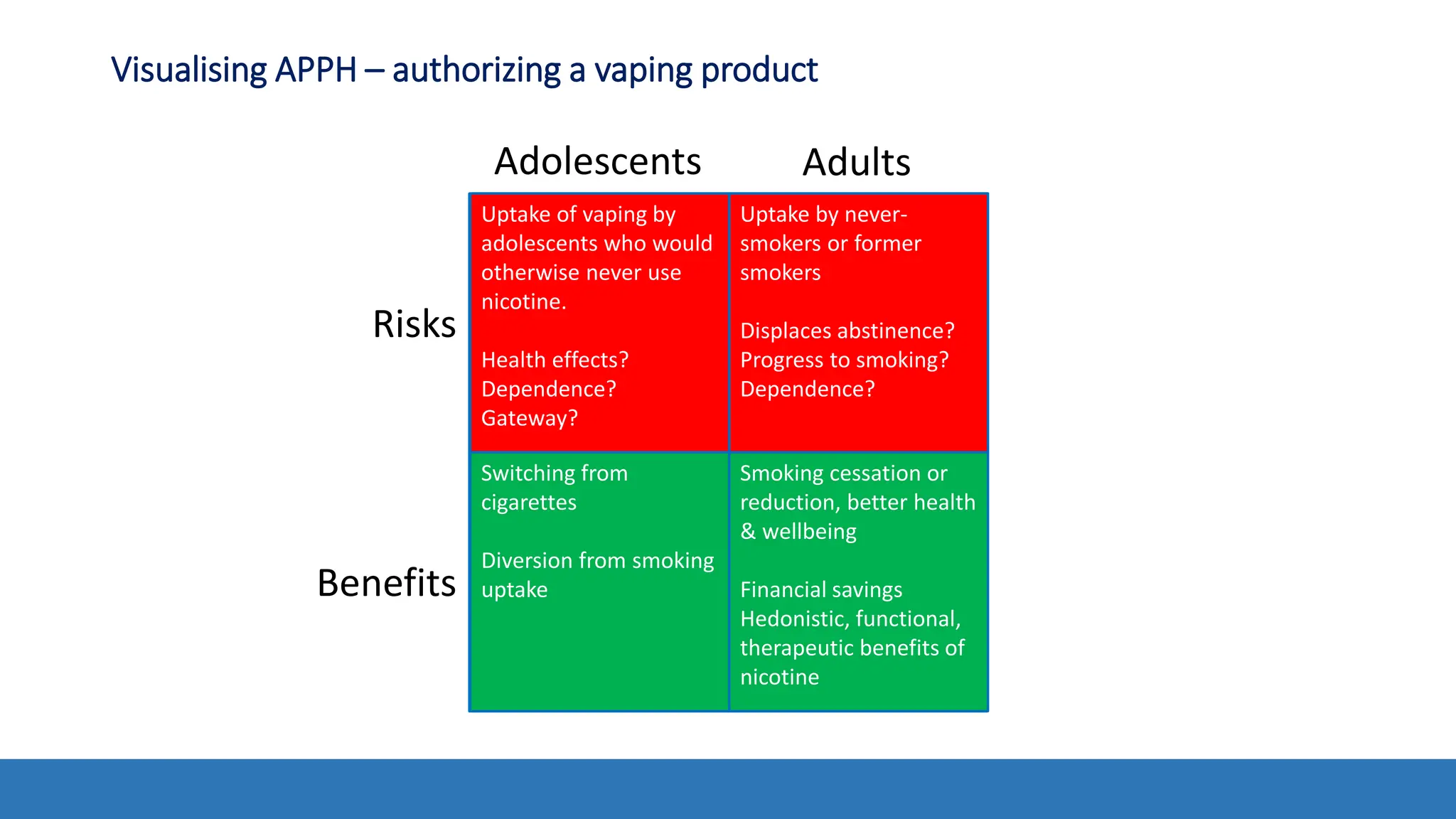
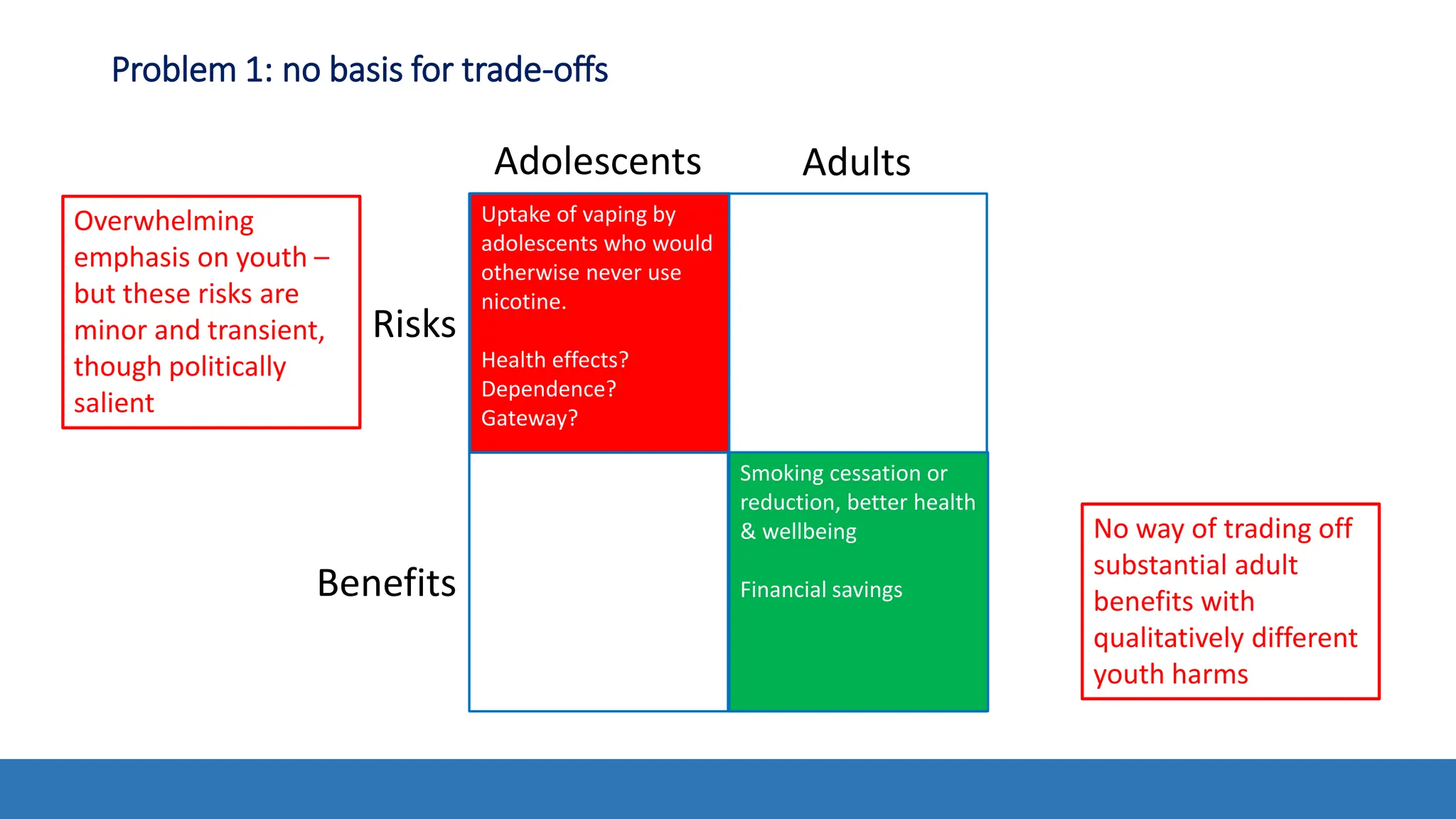
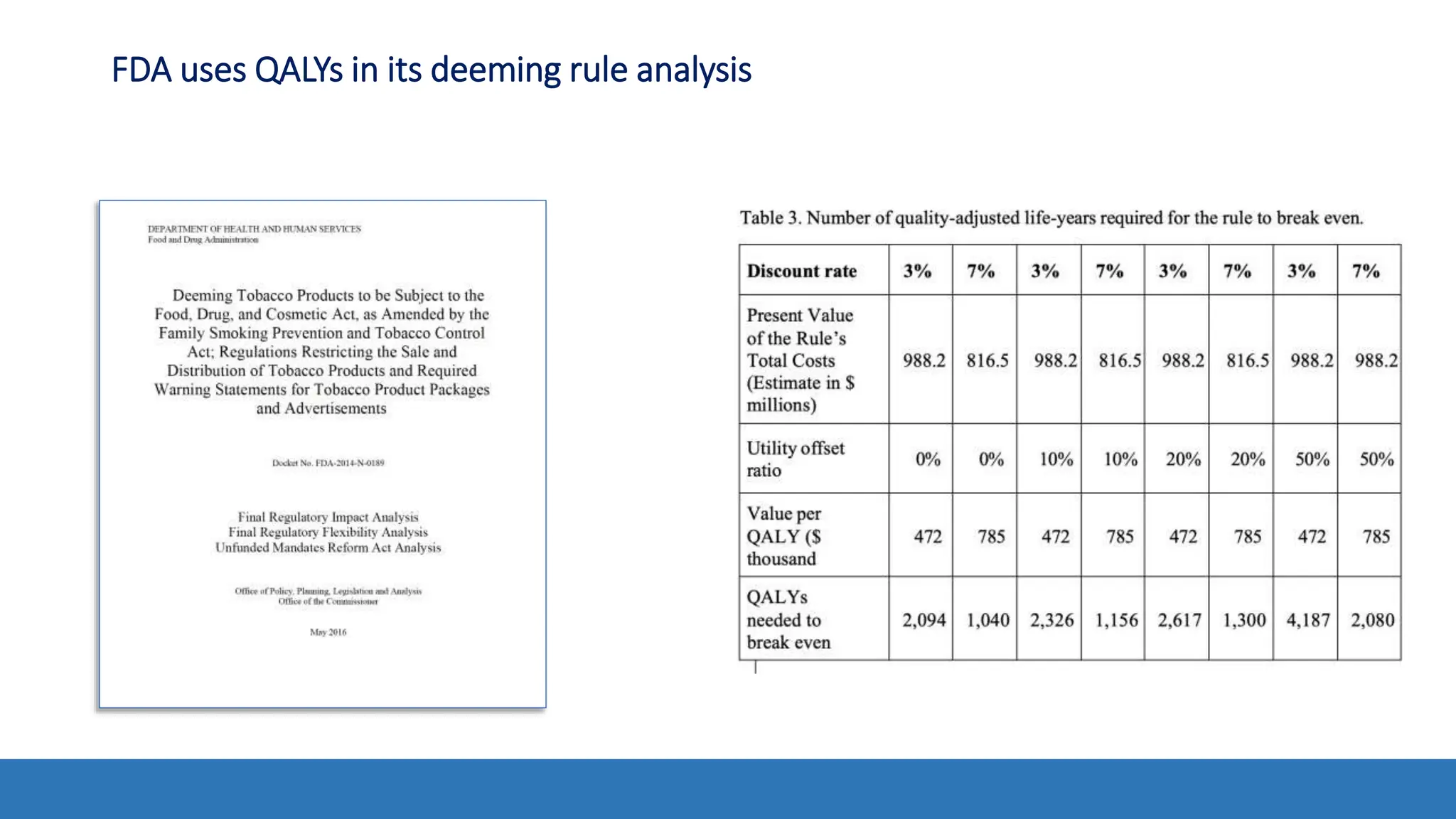
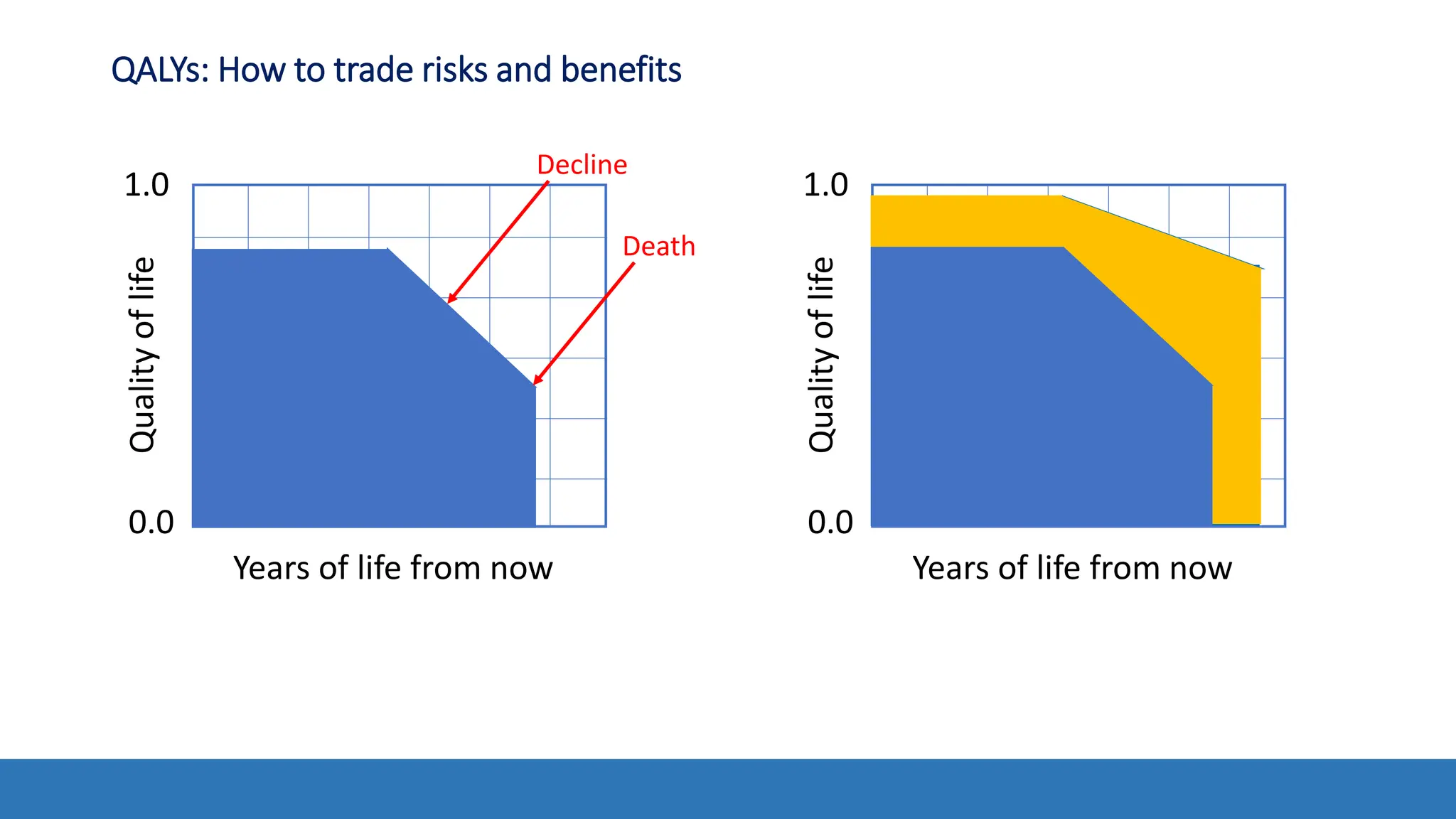
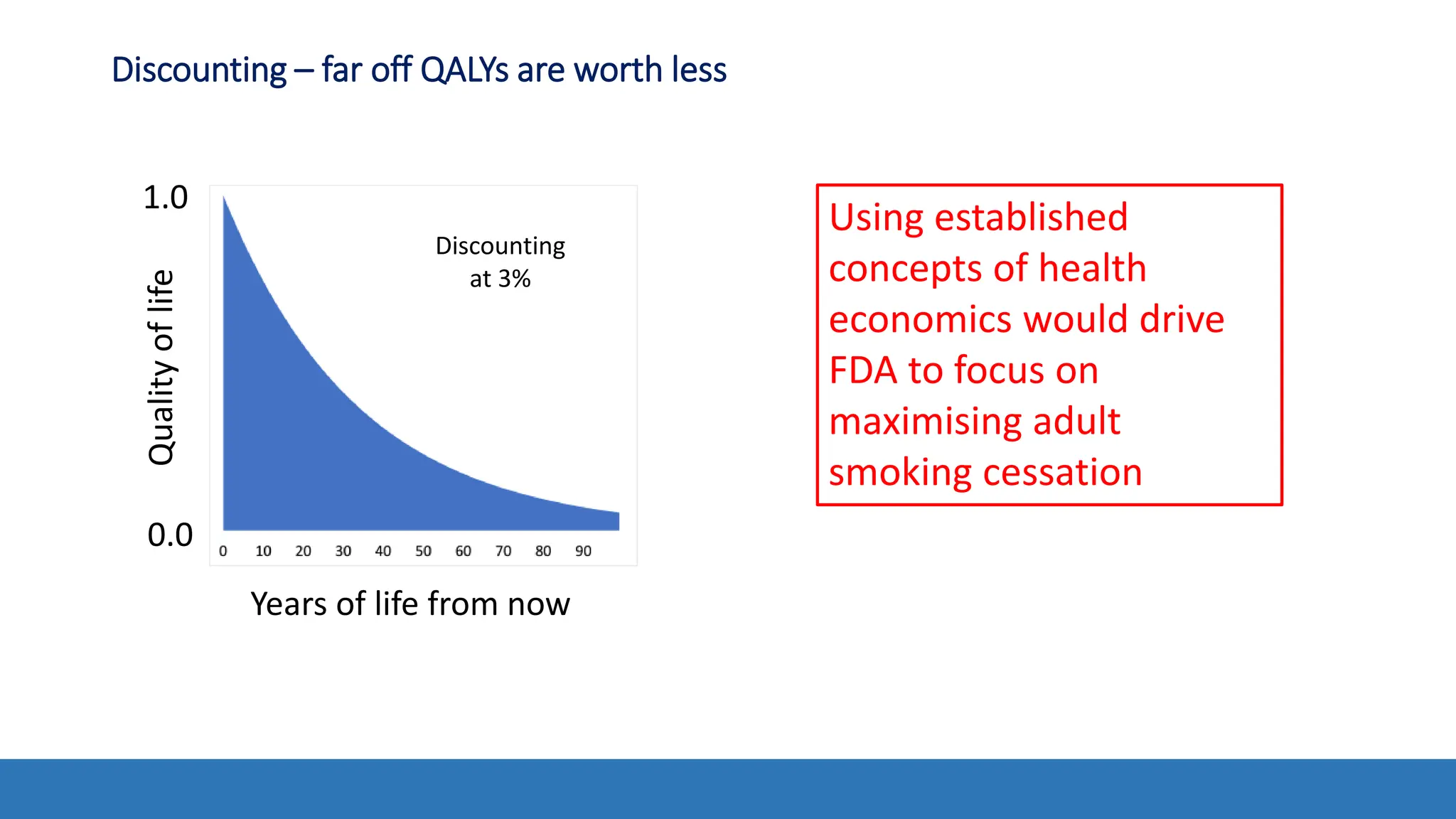
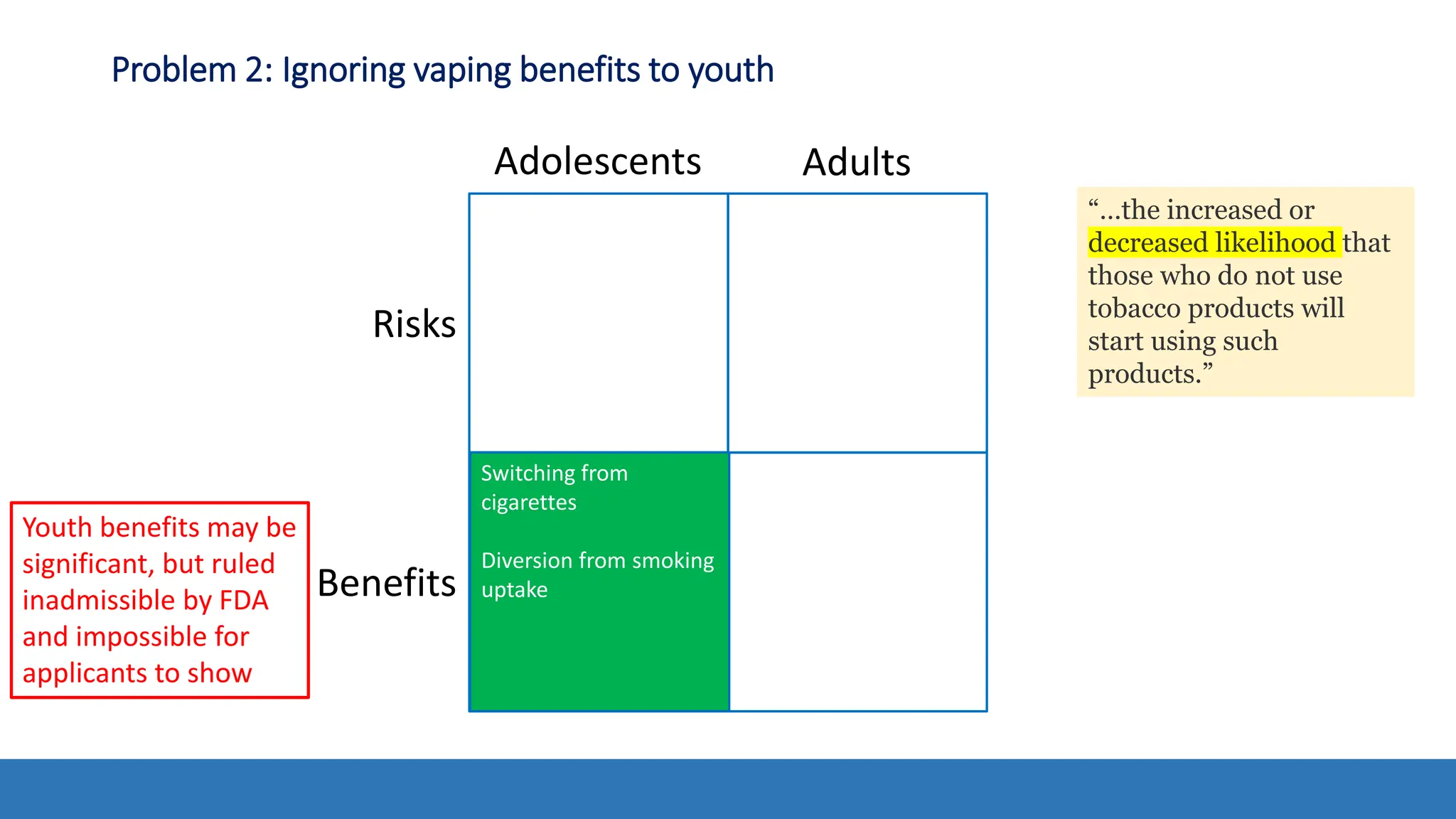
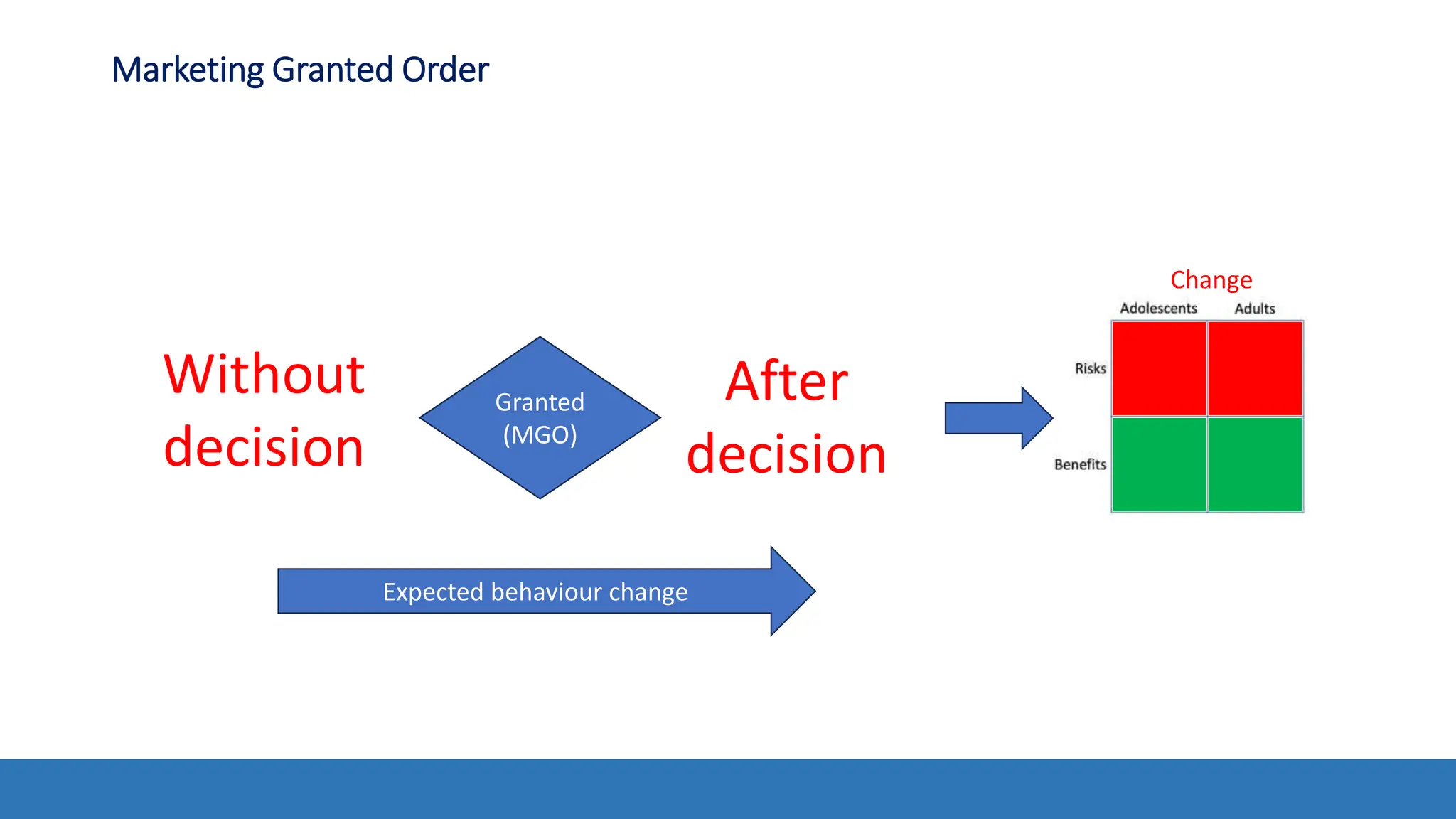
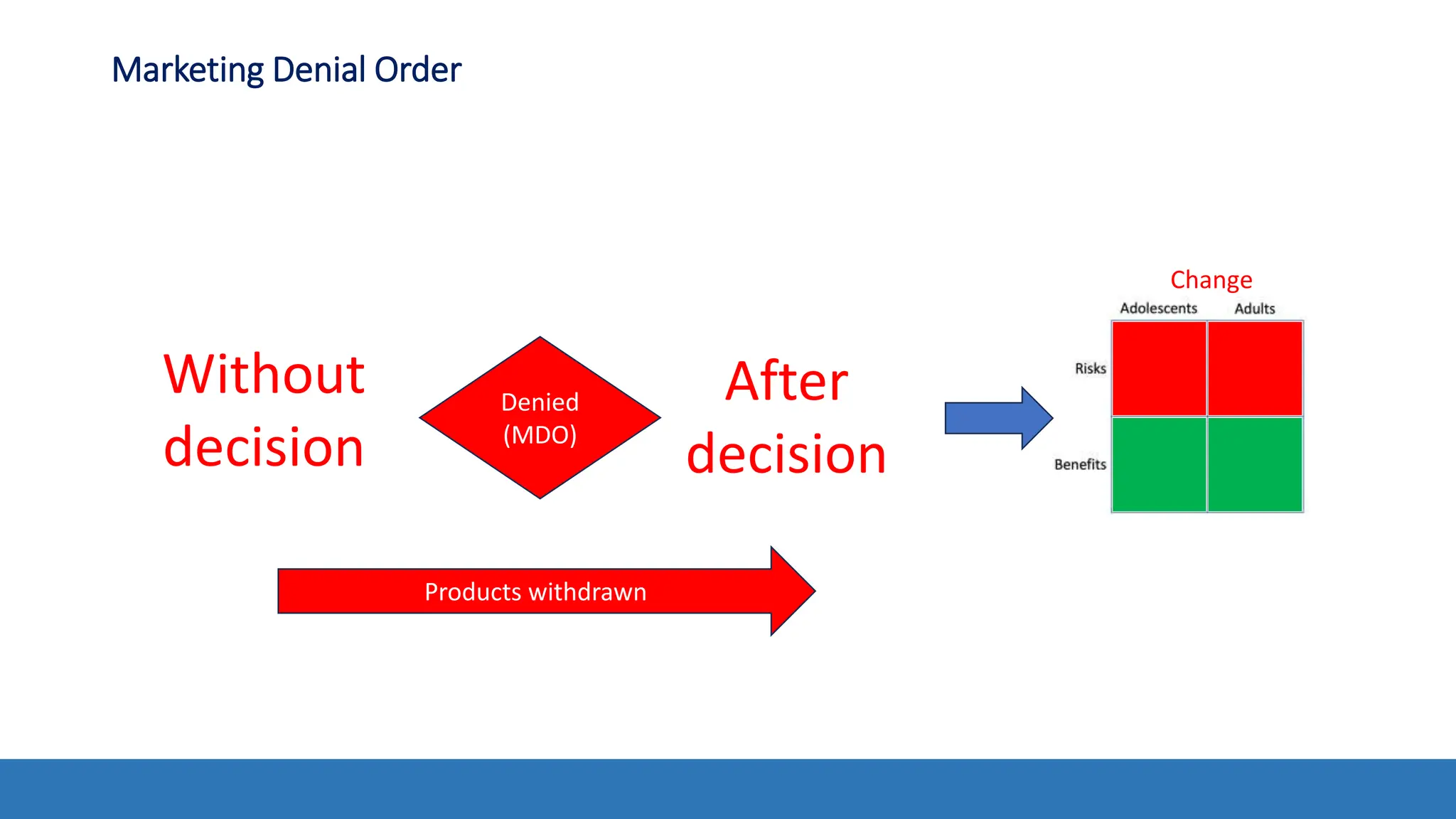
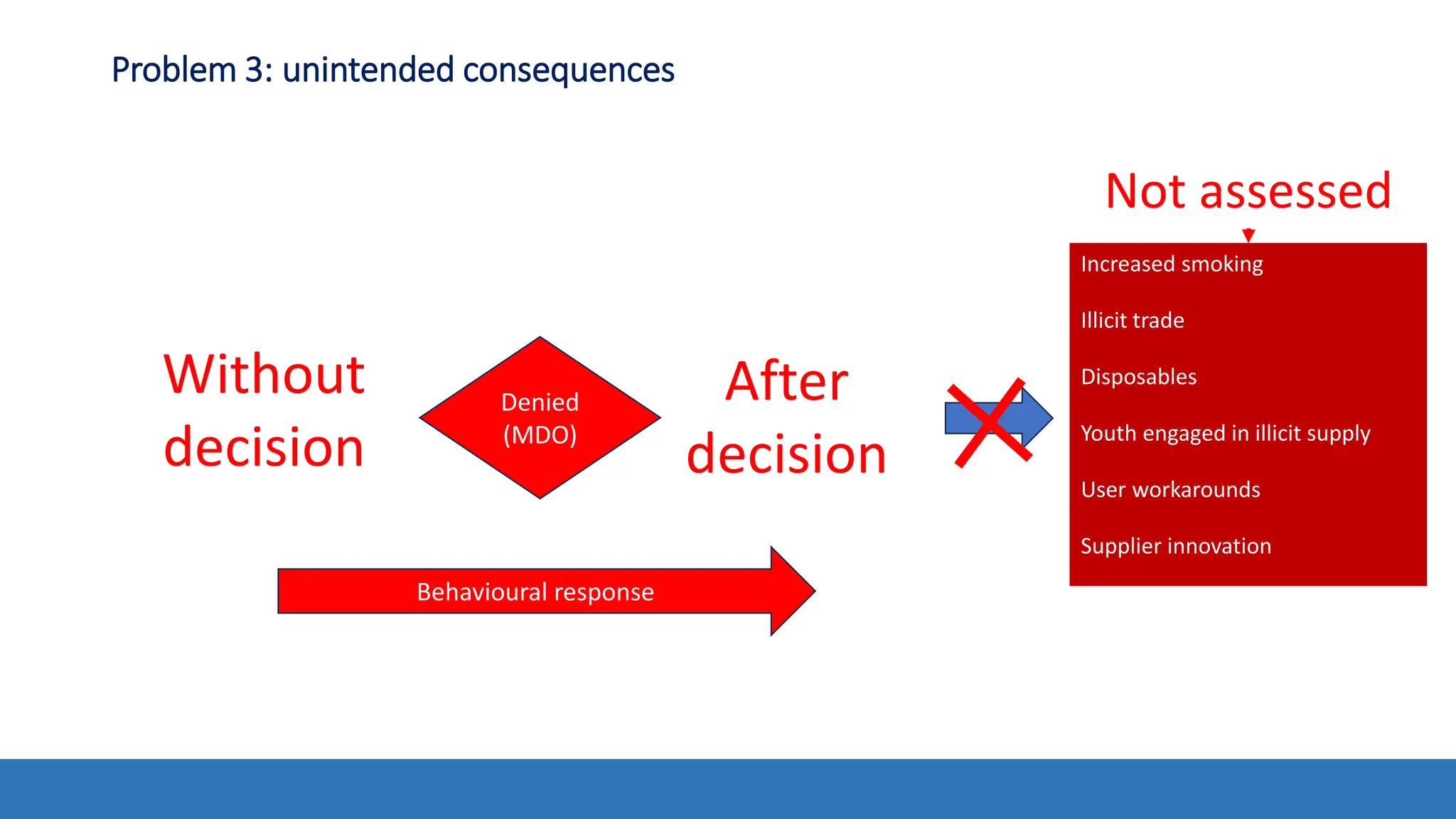
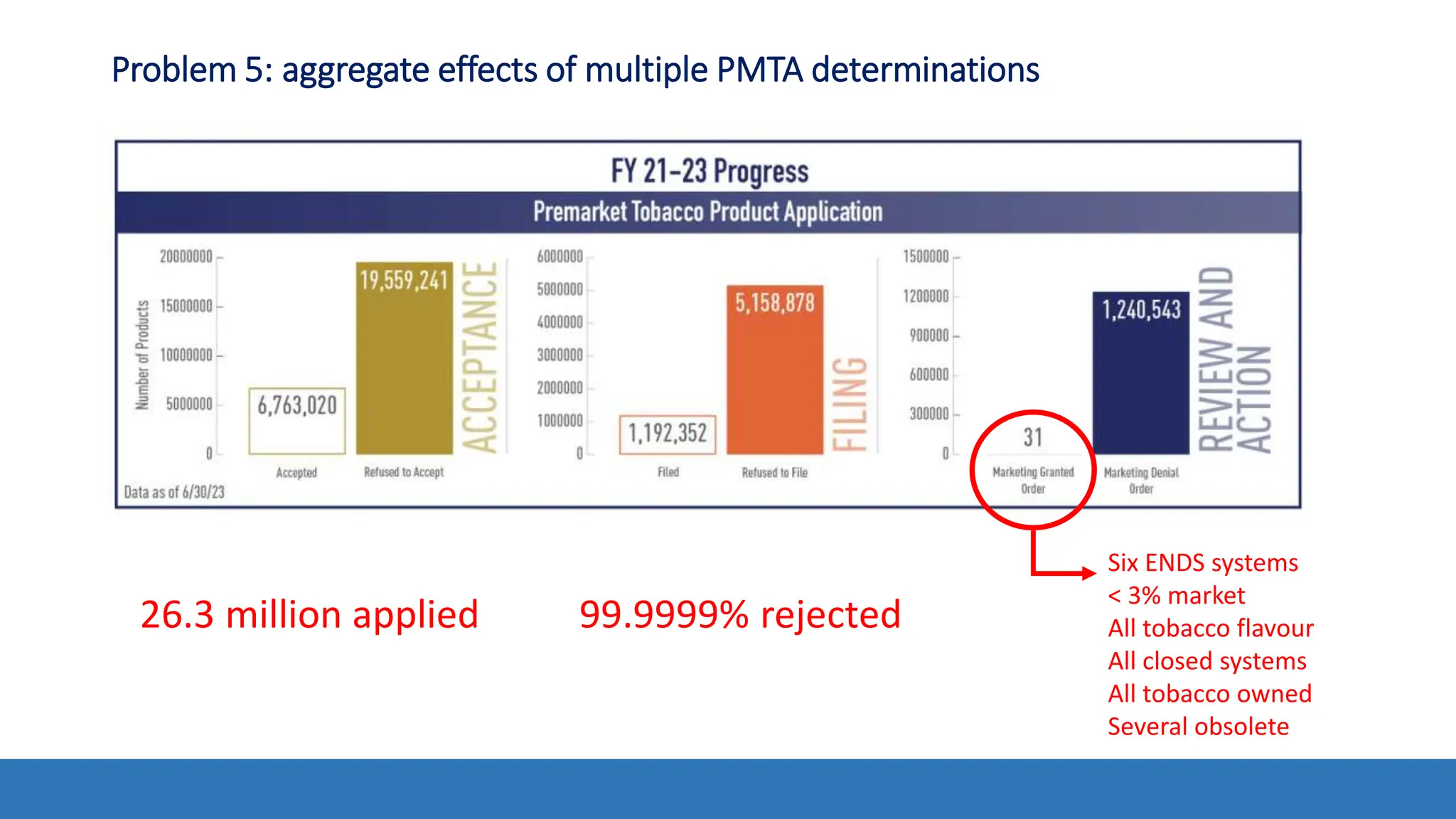
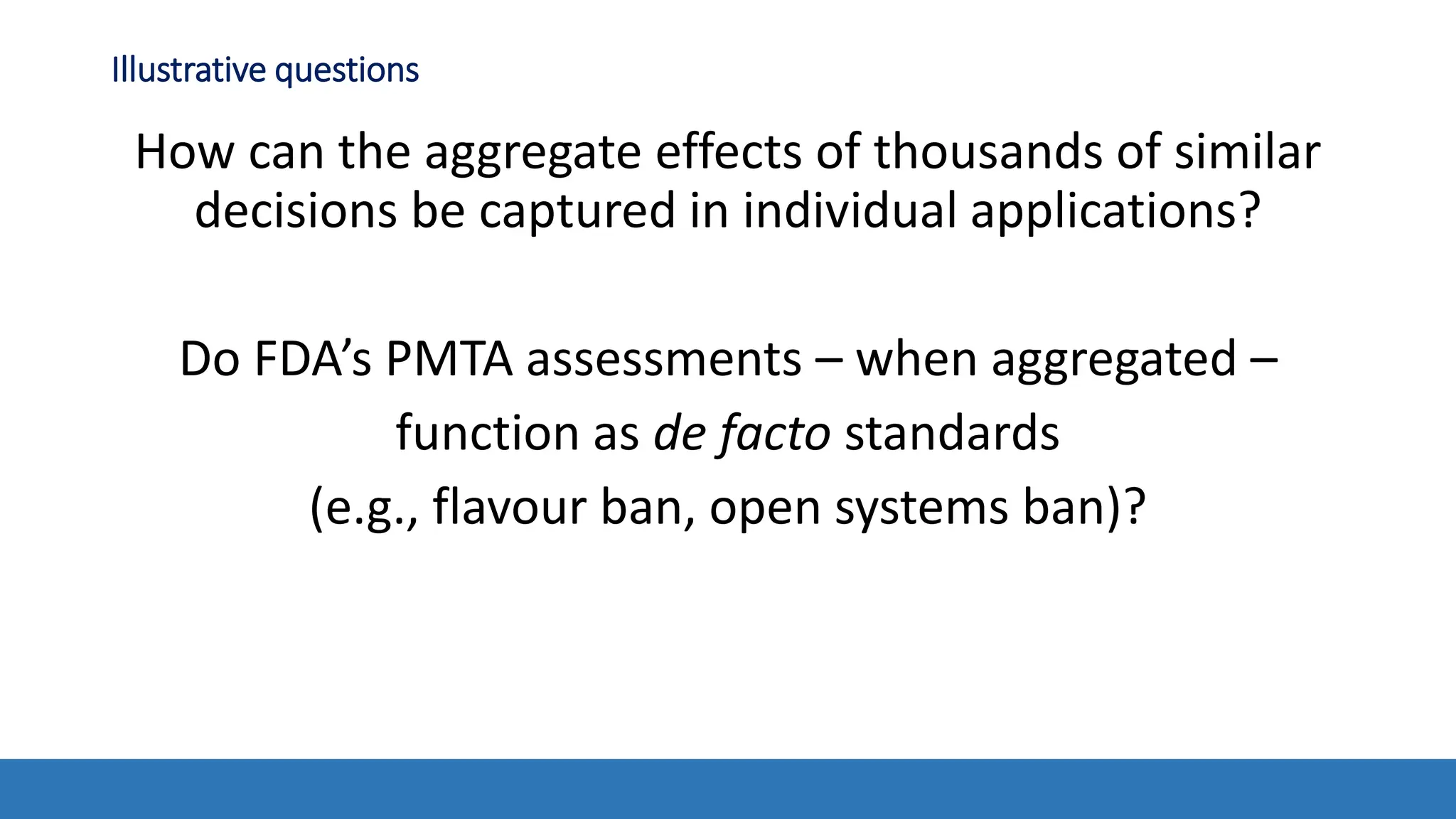
The document discusses the Appropriate for the Protection of Public Health (APPH) standard as outlined in Section 910 of the Federal Food, Drug, and Cosmetic Act, focusing on the evaluation of new tobacco products based on the risks and benefits to the overall population. It highlights various challenges and considerations related to the assessment of tobacco products, including the impact on youth vaping and adult smoking cessation, and suggests potential solutions for addressing individual and population risks. There is an emphasis on the need for comprehensive market surveillance and post-market monitoring to manage unintended consequences of product regulation.