Rutherford slide
•
1 like•350 views
Trial of a PCSK9 inhibitor in heterozygous familial hypercholesterolemia
Report
Share
Report
Share
Download to read offline
Recommended
Following the Evidence: LDL-C as a Path to Reducing Cardiovascular Events—How...

Following the Evidence: LDL-C as a Path to Reducing Cardiovascular Events—How...PVI, PeerView Institute for Medical Education
More Related Content
Similar to Rutherford slide
Following the Evidence: LDL-C as a Path to Reducing Cardiovascular Events—How...

Following the Evidence: LDL-C as a Path to Reducing Cardiovascular Events—How...PVI, PeerView Institute for Medical Education
Similar to Rutherford slide (20)
Target ldl levels in extreme high risk acs. acheiving the goal

Target ldl levels in extreme high risk acs. acheiving the goal
Design and Synthesis of some Pyrimidine as DPP-IV Inhibitors

Design and Synthesis of some Pyrimidine as DPP-IV Inhibitors
UPDATE ON THE PCKS9 INHIBITION TO LOWER LDL CHOLESTEROL

UPDATE ON THE PCKS9 INHIBITION TO LOWER LDL CHOLESTEROL
DIABETES Y ENFERMEDAD CARDIOVASCULAR 23NOVIEMBRE18 

DIABETES Y ENFERMEDAD CARDIOVASCULAR 23NOVIEMBRE18
Linagliptin Endocrinologist Prespective - Case.pptx

Linagliptin Endocrinologist Prespective - Case.pptx
Ezzahti sijbrands mulder roeters van lennep familial hypercholesterolemia new...

Ezzahti sijbrands mulder roeters van lennep familial hypercholesterolemia new...
Following the Evidence: LDL-C as a Path to Reducing Cardiovascular Events—How...

Following the Evidence: LDL-C as a Path to Reducing Cardiovascular Events—How...
Ueda2016 new horizon in the management of dyslipidemia - diaa ewais

Ueda2016 new horizon in the management of dyslipidemia - diaa ewais
Resultados de la inhibición de PCSK9: superando los límites

Resultados de la inhibición de PCSK9: superando los límites
More from Marilyn Mann
More from Marilyn Mann (20)
Comparative effectiveness randomized trial to improve stroke care delivery c...

Comparative effectiveness randomized trial to improve stroke care delivery c...
ODYSSEY Outcomes: Cardiovascular Outcomes with Alirocumab after ACS

ODYSSEY Outcomes: Cardiovascular Outcomes with Alirocumab after ACS
Pcsk9 loss of-function genetic variant is associated with pre-diabetes and di...

Pcsk9 loss of-function genetic variant is associated with pre-diabetes and di...
Alirocumab effect on new-onset or worsening diabetes, blood glucose, and HbA1c.

Alirocumab effect on new-onset or worsening diabetes, blood glucose, and HbA1c.
Circ cardiovasc qual outcomes 2013 sep 6(5) 507 8, figure

Circ cardiovasc qual outcomes 2013 sep 6(5) 507 8, figure
Ph rma principlesforresponsibleclinicaltrialdatasharing

Ph rma principlesforresponsibleclinicaltrialdatasharing
Ema scientific conclusions and grounds for refusal of mipomersen

Ema scientific conclusions and grounds for refusal of mipomersen
Oxman et al a surrealistic mega analysis of redisorganization theories

Oxman et al a surrealistic mega analysis of redisorganization theories
Glasziou taking healthcare interventions from trial to practice

Glasziou taking healthcare interventions from trial to practice
Ioannidis why science is not necessarily self correcting

Ioannidis why science is not necessarily self correcting
Recently uploaded
Cara Menggugurkan Kandungan Dengan Cepat Selesai Dalam 24 Jam Secara Alami Bu...

Cara Menggugurkan Kandungan Dengan Cepat Selesai Dalam 24 Jam Secara Alami Bu...Cara Menggugurkan Kandungan 087776558899
Recently uploaded (20)
Exclusive Call Girls Bangalore {7304373326} ❤️VVIP POOJA Call Girls in Bangal...

Exclusive Call Girls Bangalore {7304373326} ❤️VVIP POOJA Call Girls in Bangal...
💚Chandigarh Call Girls Service 💯Piya 📲🔝8868886958🔝Call Girls In Chandigarh No...

💚Chandigarh Call Girls Service 💯Piya 📲🔝8868886958🔝Call Girls In Chandigarh No...
Chennai ❣️ Call Girl 6378878445 Call Girls in Chennai Escort service book now

Chennai ❣️ Call Girl 6378878445 Call Girls in Chennai Escort service book now
💚Call Girls In Amritsar 💯Anvi 📲🔝8725944379🔝Amritsar Call Girl No💰Advance Cash...

💚Call Girls In Amritsar 💯Anvi 📲🔝8725944379🔝Amritsar Call Girl No💰Advance Cash...
Chandigarh Call Girls Service ❤️🍑 9809698092 👄🫦Independent Escort Service Cha...

Chandigarh Call Girls Service ❤️🍑 9809698092 👄🫦Independent Escort Service Cha...
Cara Menggugurkan Kandungan Dengan Cepat Selesai Dalam 24 Jam Secara Alami Bu...

Cara Menggugurkan Kandungan Dengan Cepat Selesai Dalam 24 Jam Secara Alami Bu...
Pune Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Pune No💰Adva...

Pune Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Pune No💰Adva...
Goa Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Goa No💰Advanc...

Goa Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Goa No💰Advanc...
Call Girls in Lucknow Just Call 👉👉8630512678 Top Class Call Girl Service Avai...

Call Girls in Lucknow Just Call 👉👉8630512678 Top Class Call Girl Service Avai...
💚Reliable Call Girls Chandigarh 💯Niamh 📲🔝8868886958🔝Call Girl In Chandigarh N...

💚Reliable Call Girls Chandigarh 💯Niamh 📲🔝8868886958🔝Call Girl In Chandigarh N...
ANATOMY AND PHYSIOLOGY OF REPRODUCTIVE SYSTEM.pptx

ANATOMY AND PHYSIOLOGY OF REPRODUCTIVE SYSTEM.pptx
❤️Chandigarh Escorts Service☎️9814379184☎️ Call Girl service in Chandigarh☎️ ...

❤️Chandigarh Escorts Service☎️9814379184☎️ Call Girl service in Chandigarh☎️ ...
💰Call Girl In Bangalore☎️7304373326💰 Call Girl service in Bangalore☎️Bangalor...

💰Call Girl In Bangalore☎️7304373326💰 Call Girl service in Bangalore☎️Bangalor...
❤️Amritsar Escorts Service☎️9815674956☎️ Call Girl service in Amritsar☎️ Amri...

❤️Amritsar Escorts Service☎️9815674956☎️ Call Girl service in Amritsar☎️ Amri...
Call Girls in Lucknow Just Call 👉👉 8875999948 Top Class Call Girl Service Ava...

Call Girls in Lucknow Just Call 👉👉 8875999948 Top Class Call Girl Service Ava...
❤️Call Girl Service In Chandigarh☎️9814379184☎️ Call Girl in Chandigarh☎️ Cha...

❤️Call Girl Service In Chandigarh☎️9814379184☎️ Call Girl in Chandigarh☎️ Cha...
Gastric Cancer: Сlinical Implementation of Artificial Intelligence, Synergeti...

Gastric Cancer: Сlinical Implementation of Artificial Intelligence, Synergeti...
Race Course Road } Book Call Girls in Bangalore | Whatsapp No 6378878445 VIP ...

Race Course Road } Book Call Girls in Bangalore | Whatsapp No 6378878445 VIP ...
Bhawanipatna Call Girls 📞9332606886 Call Girls in Bhawanipatna Escorts servic...

Bhawanipatna Call Girls 📞9332606886 Call Girls in Bhawanipatna Escorts servic...
Rutherford slide
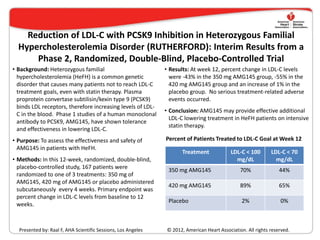
- 1. Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD): Interim Results from a Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial • Background: Heterozygous familial • Results: At week 12, percent change in LDL-C levels hypercholesterolemia (HeFH) is a common genetic were -43% in the 350 mg AMG145 group, -55% in the disorder that causes many patients not to reach LDL-C 420 mg AMG145 group and an increase of 1% in the treatment goals, even with statin therapy. Plasma placebo group. No serious treatment-related adverse proprotein convertase subtilisin/kexin type 9 (PCSK9) events occurred. binds LDL receptors, therefore increasing levels of LDL- • Conclusion: AMG145 may provide effective additional C in the blood. Phase 1 studies of a human monoclonal LDL-C lowering treatment in HeFH patients on intensive antibody to PCSK9, AMG145, have shown tolerance statin therapy. and effectiveness in lowering LDL-C. • Purpose: To assess the effectiveness and safety of Percent of Patients Treated to LDL-C Goal at Week 12 AMG145 in patients with HeFH. Treatment LDL-C < 100 LDL-C < 70 • Methods: In this 12-week, randomized, double-blind, mg/dL mg/dL placebo-controlled study, 167 patients were 350 mg AMG145 70% 44% randomized to one of 3 treatments: 350 mg of AMG145, 420 mg of AMG145 or placebo administered 420 mg AMG145 89% 65% subcutaneously every 4 weeks. Primary endpoint was percent change in LDL-C levels from baseline to 12 Placebo 2% 0% weeks. Presented by: Raal F, AHA Scientific Sessions, Los Angeles © 2012, American Heart Association. All rights reserved.
