Vtesse & the Patient Community - Working Together to Bring New Solutions for NPC
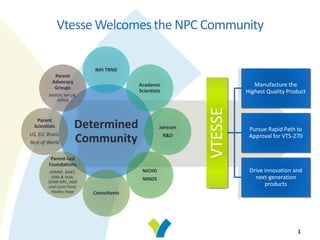
- 1. Vtesse Welcomes the NPC Community Determined Community NIH TRND Academic Scientists Janssen R&D NICHD NINDS Consultants Parent-Led Foundations APMRF, DART, Hide & Seek, SOAR-NPC, Addi and Cassi Fund, Hadley Hope Parent Scientists US, EU, Brazil, Rest of World Parent Advocacy Groups NNPDF, NP-UK, INPDA VTESSE Manufacture the Highest Quality Product Pursue Rapid Path to Approval for VTS-270 Drive innovation and next-generation products 1
- 2. 2 Good evening. My name is Ben Machielse, I am the CEO of Vtesse. Thanks so much for participating in this webinar. It is an honor to host this webinar. I'm going to take a step back and think about how this cyclodextrin project came to fruition. Actually it all started as an initiative fueled by parents, supported by parent advocacy groups and parent- led foundations. There was significant involvement of many, many academic scientists, NIH TRND and NIH NCATS really played a major role. We should not forget to mention Johnson & Johnson who actually supplied the drug. We are honored that we actually can take this program to the next step and focus on getting this drug approved.
- 3. CONFIDENTIAL & PROPRIETARY VTESSE & THE PATIENT COMMUNITY WORKINGTOGETHERTOBRINGNEWSOLUTIONSFORNPC 2015
- 4. Webinar Goals • Answer questions and understand parent needs to make participation in a pivotal trial as easy as possible • Share information on the path to drug approval and pivotal trial • Learn about Vtesse • Establish communication channel with Vtesse • Agree on a mutual sense of urgency to demonstrate safety and efficacy of VTS-270 to ensure wider availability • Clinician perspective on the drug development process • Why drug development process is necessary for NPC community • Parent perspective of having a child treated with VTS-270 We will plan future webinars to inform and update the community 4
- 5. CONFIDENTIAL & PROPRIETARY INTRODUCTION Ben Machielse, Drs. Vtesse President & CEO 5
- 6. 6 I want to emphasize before we go into the presentation that this is not a patient recruitment seminar. We are actually trying to learn from the parents and families, what their thoughts are about our activities, and about the trial. As I mentioned on January 7th, we are very much interested in listening and learning from your perspective to actually move this program forward. We hope that we can establish an effective communication channel with you, and hopefully we can all agree on the mutual sense of urgency to demonstrate the safety and efficacy of VTS- 270 to ensure the widest availability possible. I am very pleased that Marc Patterson, as a clinician, will share his perspective on the need for drug development and why a drug development process is necessary for the NPC community to get the drug approved. Also, Phil Marella will speak on his perspective and his experience with his son Andrew, who is being treated with VTS-270 in the NIH Phase I trial. As I said before we plan future webinars to inform and update the community.
- 7. Vtesse Core Values • We care about the NPC community • We are committed to help NPC patients and their families • Singular focus – develop and seek regulatory approval for VTS-270 (2- hydroxypropyl-b-cyclodextrin) to treat NPC Put NPC patients and families first • With you, we will do everything possible to answer how well VTS-270 works • We operate with a sense of urgency Think beyond limits • We have a solution-oriented mindset • We will listen and communicate openly where we can • We aspire to deliver on our promises Hold ourselves responsible 7 A privilege to serve this community by developing and seeking approval for VTS-270 for the treatment of NPC
- 8. 8 First, just a little bit about Vtesse. When we started working on this opportunity what I sensed was the commitment of the community to actually do whatever is possible to seek a solution for their children. We are committed to help the NPC patients and their families. As a company, we focus on VTS-270 only, and really accelerate that to seek approval for VTS-270 as soon as possible. We think beyond limits. With you, we will do everything possible to answer how well VTS-270 works. Having good data and understanding how VTS-270 works is absolutely essential to develop the best future treatment paradigm for these patients. We operate with a sense of urgency. We started January 7. We have had our first interactions with the regulators already, and we have hired our CRO (Clinical Research Organization who will run our clinical trial), and we are really marching forward to start the study as soon as possible.
- 9. 9 As a team we had hold ourselves responsible. We have a solution-oriented mindset. We know that this will not be an easy project, but with our shared commitment to find a solution for the NPC children we think and we hope that we can contribute to a solution. We will listen and communicate openly where we can. I hope you understand that we are still in active discussions with the regulators, and sometimes it is difficult to share everything because some things are simply not yet decided, or not yet approved. To protect the open dialogue with the regulators, we also sometimes have to be a bit cautious. We aspire to deliver on our promises. We hate to make commitments we cannot fulfil. Sometimes it will lead to us being a little bit careful on what we share because sometimes we are not yet sure, but I promise you we will always be honest. It is a privilege to serve this community, we see it as a challenge and we hope to contribute to the development and the approval of VTS-270 with the treatment of NPC.
- 10. Introductions Vtesse Team • Our team has developed and sought approval for 20 compounds • Two major R&D functions – clinical research and technical operations • Clinical research: launch a global pivotal clinical study for VTS-270 • Technical operations: improve the drug and method of administration Our Panel Today • Ben Machielse, Vtesse • Marc Patterson, Mayo Clinic • Paul Gissen, GOSH (EU Session) • Liz Berry-Kravis, Rush University (US session) • Phil Marella, Parent • Will Evans, Parent (introductions, EU session) • Participating: Ravi Venkataramani, Sarah Frech, Jehan Rowlands, Carol Tressler, Siren Interactive 10
- 11. I’ll pass the torch to Will Evans for introductions. Will: Thank you. So I’ll start by introducing myself, many of you may have met me. I’m Will Evans, the father of Sam Evans. He’s 7 and he has Niemann-Pick Type C. I also have 2 other children who are unaffected. I’m a trustee with Niemann-Pick UK and in my day job I’m also a General Practitioner, a family physician in England. We live in Northern England. So it’s important for me to give a brief intro and tell you a little bit about things before we hear more about things we’ve been looking forward to. I have to share with you the excitement of hearing the announcement of the long awaited and hopeful cyclodextrin trial. I know many people on this call, myself included, have spent a large amount of time finding out about what’s going on with the Phase I trial, with the work done before that - the preclinical work, and all the excitement that came with the impressive results that we’ve seen. We’ve also, myself included, explored compassionate access and other options to access this therapy; but, the key thing was, and certainly what I wanted was to get a clinical trial in Europe where we can get a definitive answer and proof that the drug hopefully works and we can all get access for people with NPC across the world. 11
- 12. Throughout the process myself as a representative of NP UK, but also the INPDA have been in contact with the NIH for a long time, and having regular teleconferences with them and also with Vtesse, the company, right from the outset of the formation of the company and actually before then. So Vtesse have actually been engaging us as a patient group in Europe as of summer of last year. And, they have been at pains to try and keep us informed of what’s going on and have listened and valued our input throughout this process to try and achieve what they truly do see as a multi-nationals trial with EU having equal footing as the US, which I am greatly encouraged by; and, I’m sure you will hear more about that later. Today’s webinar is a start and further demonstration of this willingness to engage with us as a patient and parent group, as well as a chance for us to learn more about Vtesse’s intentions. It’s a way to learn more about us as a patient group in Europe and how they can help us facilitate this trial and address some of our needs as well. There are things they can share and some they can’t because it’s still a process as they are still deciding what needs to be done and they are still in contact with regulators and centers. So I think it’s important to emphasize, and we would hopefully gain lots of information today; but, maybe not as much as we first hoped. 12
- 13. The key thing to say is that we are all painfully aware of the urgency of this. We all have loved ones affected by this. I also feel from conversations with Vtesse that they appreciate this; and, they really do value and know that this is something they can’t hang about with, and they need to get answers quickly so we can definitely know if cyclodextrin works, and therefore hopefully have an approved compound that we can all access for our patients. I look forward to the discussions. 13
- 14. 14 Ben: Let me talk about the Vtesse team. Our team has developed jointly and sought approval for over 20 compounds which are currently used in the field. We understand the interactions with the regulators, and we understand also how to develop a label which is meaningful for the patients who actually will receive the drug in the future. As a company, we have two major R&D functions: clinical research led by Sarah Frech, that is focused on the launch of the pivotal clinical study. They have developed the protocols, they have sought scientific advice, and they are actively interacting with the CRO to accelerate the development of the clinical plan at the highest pace possible. Then we have a technical operations function led by Allan Darling. Their focus is to further improve the drug and also improve on the method of administration. We see this as part of our long-term commitment to continue to optimise the current drug cyclodextrin, and possibly develop new drugs for the treatment of NPC.
- 15. 15 Our panel today consists of myself, Marc Patterson from the Mayo Clinic, you probably all know him but Marc is on our SAB and has been working on NPC for many many years and he is considered one of the major experts in the world on NPC. We also have here Liz Berry-Kravis from Rush University. She is also a neurologist and she treats some patients under iIND, and has tremendous experience with the NPC patients; and, we are collaborating with Liz to learn where we can. Also, Phil Marella, he will present as a parent on his experience in the Phase I trial. Last but not least, and I forgot to put them on the list and I take the blame for that, Denny Porter is here also as a panellist to share his experience. Denny Porter of NIH with his team has driven forward the development of cyclodextrin for the treatment of NPC to a point where we now can take it to a pivotal trial. We are building on the foundation which has been laid by the parents, by the iIND parents, and by the work at NIH and academic institutions to design the best possible study. Other people participating are Ravi Venkataramani, our Chief Business Officer, Sarah Frech, Head of Clinical, Jehan Rowlands, Head of Regulatory Affairs, Carol Tressler, our Office Manager, and Siren Interactive.
- 16. Where We Stand Today • We have the right ingredients to drive this program forward and seek approval for VTS-270 to treat NPC • Licensed rights from the National Institutes of Health (NIH) • Transferred Investigational New Drug (IND) application and the Orphan Drug Designations for US and EU • Raised money from investors to conduct the pivotal clinical trial • Completed initial interactions with US and EU regulators • Our immediate goal: initiate a pivotal clinical study to seek approval for VTS-270 • Longer term vision: evaluate potential second-generation drug • Method of administration that decreases burden to patient 16
- 17. 17 Where do we stand today? We have combined the right ingredients to drive this program forward and seek approval for VTS-270. We have licensed the rights to cyclodextrin and other compounds for the treatment of NPC from the National Institute of Health, and that gives us a wealth of data which helps us to analyze the historical data, and the data on Phase I which allows us to optimize the design of the clinical study. We transferred the IND (the investigational new drug application) and the Orphan Drug Designations to Vtesse for the US and EU, and this will allow us to actively interact with regulators to discuss our clinical development plan. Absolutely important too is that we raised enough money from investors to conduct a pivotal trial and we are very confident that we are very well equipped from a resource, knowledge, and know-how point of view to start and complete this clinical trial. As said before, our one single goal is to initiate the pivotal clinical study to seek approval. The longer term vision is evaluate, based on what we learn from cyclodextrin, second generation drugs and improve the method of administration so that we decrease the burden of the patients in case we demonstrate that cyclodextrin works.
- 18. VTS-270 About VTS-270 • A formulation of 2-hydroxypropyl- b-cyclodextrin (HPbCD) • Extensive safety profile in multiple applications • Deep preclinical knowledge • HPbCDs are complex mixtures • Currently in Phase I testing in the US Efficacy • Strong efficacy results in mice and cat models of NPC disease • Prevents cerebellar dysfunction such as ataxia • Preserves Purkinje cells • Prolongs lifespan • Route of administration is important in treating the neurological disease • In cats, administration directly into the brain improves neurological disease more than injecting into the body • Intrathecal/intracranial is better than intravenous/subcutaneous for neurological disease 18
- 19. 19 What is VTS-270? VTS-270 is the formulation of 2-hydroxypropyl-beta-cyclodextrin. It has been studied over many, many years. It has an extensive safety profile in multiple indications. Fortunately, we have a very deep preclinical knowledge of 2-hydroxypropyl-beta- cyclodextrin that we are using in our trial. We do realize that cyclodextrins are complex mixtures, and one mixture is not necessarily the same as the other mixtures. Currently VTS-270 is in Phase I testing in the US at the NIH site.
- 20. 20 From an efficacy point of view, we are really optimistic because the efficacy results in mice and cat models of NPC show that there are clear indications that VTS-270 prevents cerebellar dysfunction such as ataxia. Also it has been demonstrated that VTS-270 preserves Purkinje cells. Very importantly in both the mice and the cat model, it is a clear prolongation of life span and that set of data in combination with what we have learned from the Phase I study and the natural history study gives us a sense of optimism. I must mention that the route of administration is very important in treating the neurological aspects of the disease. In cats, administration directly into the brain improves neurological disease more than injecting it into the body. Therefore we have elected to administer this drug intrathecally (lumbar puncture). Based on the data in the preclinical study as generated by many academic institutions, it is better than intravenous administration for the treatment of the neurological disease. Having said that, this is an initial introduction of where we stand today, who we are as a company and our initial plan. I would like to actually pass the torch to Marc Patterson who is going to share a clinician’s perspective.
- 21. A CLINICIANS’ PERSPECTIVE Marc C. Patterson, MD, FRACP, FAAN, FANA Professor of Neurology, Pediatrics and Medical Genetics Mayo Clinic Children’s Center 21
- 22. 22 Thank you very much. Just to introduce myself to people who may not know me, I am a Pediatric Neurologist. I got involved in this field about 25 years ago when I was at NIH. I had the privilege of being involved in the first randomized study in Niemann-Pick C which was a study of cholesterol lowering agents subsequently in the study of Miglustat. I have had the greatest privilege of caring for many, many patients over many years now. So, I am as eager as everyone else that we have the best and the safest treatments available to treat children and adults who are afflicted by this disease.
- 23. Unmet needs and challenges in NPC: April 2015 • No approved disease modifying therapy in the US • Delayed diagnosis – significant disease burden before interventions can be pursued • Unclear which measures will be accepted globally by regulators as evidence of efficacy of treatment • Animal studies provide strong data supporting benefit from 2- hydroxypropyl-b-cyclodextrin (cyclodextrin) in NPC - but there is no controlled data yet on efficacy of cyclodextrin in humans 23
- 24. 24 I just want to take a moment to talk about the unmet needs and challenges in Niemann- Pick C in April of 2015. For those of us in the US we have no approved disease modifying therapy, and this is a problem. There is a therapy which is approved elsewhere which has shown evidence of efficacy, but because it is not approved by the regulatory agencies it is not freely available to everyone, so this is a considerable problem. An even bigger problem is the delayed diagnosis. A number of studies have been done in different centers, and typically the average delay in diagnosis for this disease is five years. In some cases, in adults it has been as much as 20 years. In fact, the oldest patient I diagnosed was 65 and had first developed symptoms at 40 years of age. This is a huge problem because it means when we have potentially effective therapies, the patients to whom we can administer these therapies already have a significant burden of disease. What that translates into is a loss of neurons. Neither cyclodextrin nor any other therapy that is proposed at the moment can replace neurons that are lost. So, it is absolutely critical that we make this diagnosis as soon as possible so that we can have the most effective interventions.
- 25. 25 Another problem which is not unique to Niemann-Pick C that is common amongst rare diseases that affect the nervous system is that it is difficult to know exactly what to measure that will demonstrate to the satisfaction of parents and academic investigators, and in some ways most importantly, regulators that the treatment is truly effective. In certain diseases such as cancers, survival is the outcome measure that is most widely used. That is not in any way appropriate for Niemann-Pick disease type C. We could never conduct a clinical trial which would give us data in any sort of reasonable time frame. So, we need to look at manifestations of the disease that can be measured objectively and consistently and which are not subject to what we might call noise. That is, variations which are not due to the progression of the disease itself, but to other factors such as drugs that are being administered to patients or into current illnesses and so on.
- 26. 26 Fortunately, thanks to the wonderful work of Denny Porter and his colleagues at NIH, we now have something that did not exist in the past when we were trying to design studies, and that is a large Natural History database which is now linked to a number of compounds which can be measured in different bodily fluids, in other words, biomarkers. This is a tremendous resource and it has really been essential to make progress in this disease. One of the most valuable biomarkers to come out of these studies has been the recognition that a specific trial Oxysterol is a very valuable diagnostic tool for Niemann- Pick C. Prior to the availability of this blood test, we needed to rely on tissue biopsies, genetic tests, both of which are expensive and cumbersome and have significant false positive and false negative rates. This is going to make a huge difference in our ability to diagnose this disease better. Of course as you have already heard from Ben, animal studies have provided very strong data supporting benefit from cyclodextrin in mice and cats, and although the drug has been administered to humans we do not have any controlled data yet on its efficacy. Let’s expand a little bit more on that.
- 27. Why pursue a controlled clinical trial? • To determine if perceived benefits in animal studies (“preclinical studies”) also occur in humans under controlled conditions • To determine if the agent is safe in humans • Both control and intervention groups studied using appropriate statistical methods are essential to ensure that variations in outcomes are related to the agent being tested, and not just chance • To provide data for regulatory approval (by the FDA in the US, different agencies in other countries) 27
- 28. 28 Really the question is why pursue a controlled clinical trial? We have seen the best results from any intervention in the animal models of Niemann-Pick C with cyclodextrin. So, it would be very reasonable for people to say why not just treat people? Why not just treat humans with this drug? The reason is that we don't know if it works in humans yet. We do know that humans, and this sounds trivial, are not mice and are not cats and there are many examples in science where promising agents produce what look to be impressive results in animals but don't translate to humans. The other big problem in is in humans the disease is very variable. It is absolutely essential that we have controls so that we can demonstrate that apparent benefit is true, and not just a result of random variation.
- 29. 29 In other words, the reason to pursue a controlled clinical trial is to determine if the perceived benefits in animal studies, these are the preclinical studies that have been referred to, also occur in humans under controlled conditions. Of course it is important to determine if the agent is safe in humans. We also have many examples in clinical studies where small numbers of humans are exposed to a drug early in the course of its development and then later when larger numbers are exposed adverse events which were not previously recognised or expected can become apparent. When we talk about controls it means that we need two groups of patients who are as closely matched as possible in terms of age and severity of disease, and other factors who can be compared. One will receive the active agent, one group will not.
- 30. 30 We know that when we are studying or performing these sorts of studies to account for variation that occurs naturally, we need to use statistical methods. The factors that have to be taken into account when designing a clinical trial are first of all the size of the effect, and so before investigators begin a trial they have to decide what they will measure, and what they will regard as a clinically significant change in that measure using that measure of effect size. It is then possible to calculate how many patients will be needed to participate in this study to have a reasonable chance of finding an effect, if it is there. And this number that is calculated, it is called the power of the study. For example, you may say that a study has an 80% power of detecting a 5% difference between the intervention and control groups. This is an absolutely critical number because in rare diseases one of the enormous problems we face is that agents which may produce clinically significant effects may not reach statistical significance because the numbers of patient studied are too small.
- 31. 31 This is called a Type 2 error in statistics. It is an incorrect or a false negative result. This is one of the reasons why selecting patients carefully, matching them as carefully as possible and limiting the variability are all absolutely essential in designing a study that will answer the question as clearly as possible. I can't over emphasize the importance of that. When you see detailed criteria for inclusion and exclusion from studies, these are not something that has been decided upon in a random fashion. This has been carefully thought out to ensure that this study is going to get the answer that is required. A very important reason to pursue a controlled clinical trial is that this is the only way that we will get data that will be acceptable for regulatory approval by the Food and Drug Administration of the United States and by the different agencies in other countries.
- 32. How drugs are developed and approved • Mission of regulatory agencies • FDA: “The mission of FDA's Center for Drug Evaluation and Research (CDER) is to ensure that drugs marketed in this country are safe and effective” • EMA: " to foster scientific excellence in the evaluation and supervision of medicines, for the benefit of public and animal health" • Regulatory agencies typically do not test drugs • Although they sometimes conduct limited research in the areas of drug quality, safety, and effectiveness to help with enforcement activities • “Clinical trials are experiments that use human subjects to see whether a drug is effective, and what side effects it may cause. ” http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ 32
- 33. 33 I wanted to say a word or two about how drugs are developed and approved. Now, I am not an expert on this by any means. There are people participating here who are, and could answer many more detailed questions; but, I have painfully learned some details of this and I will certainly share them with you. First of all, what is the mission of the regulatory agencies? The Food and Drug Administration states that the mission of the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) is to ensure that drugs marketed in the United States are safe and effective. Now, the European Medicines Agency has a slightly different statement. They state that their mission is to foster scientific excellence in the evaluation and supervision of medicines for the benefit of public and animal health.
- 34. 34 Now, whilst it is true that in many situations, the evaluations of these two agencies will coincide, and they are beginning to cooperate more and more, they are not the same. They have their own rules and requirements. When conducting an international or global study, which is really important for rare diseases such as Niemann-Pick C, one has to take into account the requirements of these different agencies. It is also important to remember that regulatory agencies do not do the pivotal drug trials themselves. Sometimes, they conduct research or fund research in terms of drug quality, safety, and effectiveness to help with their enforcement activities, but they typically do not fund the pivotal trials. I want to emphasize this final statement, clinical trials are experiments that use human subjects to see whether a drug is effective and what side effects it may cause. I emphasize this, they are experiments. You can't refer to the drug as a treatment until you have evidence that it actually does something in humans.
- 35. 35 It is very important that I think we use this language carefully in talking about the experimental agent or the experimental drug because if we call it a treatment, people tend to think somebody is getting treatment and somebody is in the control, but it is not. Both are participating in an experiment. In fact, the way I believe this trial will be designed, everyone will have the opportunity to receive the interventional agent after a period of time; but, I can't over emphasize the importance that clinical trials are experiments.
- 36. Regulations – why expert guidance is needed 36
- 37. 37 I do not expect you to be able to read this slide unless you have much better vision than I do; but, it is just to give you a very graphic sense of the complexity of this process. The drug regulatory process is a thicket of rules and regulations and this is not something for amateurs to do. You need professionals such as those who are involved in Vtesse who have already passed along this way who know how to negotiate these agencies. They are very strict with requirements. If you go to these web pages, you will see that each one of those blue lines represents a link that links on many pages, many regulations, many forms, and these all have to be right. These people are worse than the IRS. You've got to get everything exactly right if you are going to move along this path correctly. This takes experience.
- 38. Why pursue drug approval? • The cost of approved drugs is (usually) covered by insurance, meaning that most patients should be able to gain access to agents regarded as safe and effective • The drug approval process will also provide a better understanding of the objective efficacy of the drug • Regulatory agencies continue to monitor drugs for safety after approval • Approval of one drug provides a road map for subsequent studies of new drugs to treat the same condition 38
- 39. 39 I guess, a final question is, if you can show that this agent is effective, why is it so important to get the drug approved? The reason is very simple. In most countries, the cost of approved drugs is covered by insurance. That means that if a drug is approved most patients should be able to gain access to agents which are regarded as safe and effective in treating their illness. Now of course, if you go through this very rigorous drug approval process, it should provide better understanding of the objective efficacy of the drug. In addition, as I mentioned before, sometimes unexpected adverse effects of drugs can occur after initial studies. The regulatory agencies will continue to monitor drugs for safety and they will detect signals and intervene if necessary. One important point is that when any agent is approved for the treatment of specific manifestations of a disease, then certain criteria are established. This provides a roadmap for subsequent drug development. We know that although cyclodextrin has provided the most impressive results in animal models of this disease, it is not a cure. These animals still succumb to their illness. They survive much longer than animals treated with other agents but they still succumb. Ultimately, cyclodextrin is not going to be the final answer, but we hope it will prove to be a very important step towards that.
- 40. 40 I just want to make a couple of closing comments. As I mentioned, this trial as proposed by Vtesse for cyclodextrin is not the first trial of drugs in Niemann-Pick C. It is not the first controlled trial. There was a randomized trial of cholesterol lowering agents that was published in 1993. We did a controlled trial of Migulsat with small numbers on a population of very sick patients without the database of clinical information and with very limited funding which was published eight years ago. Now, the unique aspect of this trial is that we have a beautifully gathered and analyzed cohort of patients from Denny Porter’s study at NIH with linked biomarkers that provides the basis for designing a clinical trial. This was never available before. There was no funding to support it. It has been done and executed in a superb way. We have this basis. We have an agent which has been very effective in animals. I think this is an exciting time for everyone in this community, but it has already been stated this is still not an easy study to do. With all of these factors in our favor, it is a tough disease that is difficult to study.
- 41. 41 It is absolutely essential that this study is designed to be as likely as it can be to show a true benefit if it exists. And so, that requires rigor; and that rigor of course, means that not everybody is going to be able to participate in the study and not everyone will qualify. But it is very important that everyone understands why this is the case. Ultimately, this is their best chance to get a clear-cut answer and we hope to have an approved therapy. I am going to conclude my remarks there but I do want to take this opportunity to thank you for the opportunity to speak.
- 42. 42 Also, if I may introduce Phil Marella, I have had the opportunity to get to know Phil and his remarkable family since their journey with Niemann-Pick C began many years ago now. Phil is a really extraordinary person as are his family members. In addition to coping with the burden of this illness, they have been extraordinary fundraisers and intellectual leaders within the patient care community. They really are one of many people who I found very inspiring over the years. Again, thank you for the opportunity to speak and I'll pass the torch to you Phil.
- 43. CONFIDENTIAL & PROPRIETARY A PARENT’S PERSPECTIVE Phil Marella Father of Dana and Andrew
- 44. 44 Thank you for that Marc. I greatly appreciate it and thank you for all that you have done for the kids and the families over the long period of time you have been helping with NPC. I will introduce myself, I am Phil Marella. I live in Connecticut with my family. Many of you know our story. For those of you that don't, two of my wife Andrea’s and my children were born with Nieman-Pick C. Sadly in 2013, we lost our daughter Dana right before her 20th birthday. Our son Andrew who also has Nieman-Pick C, is going on 16 now and he is actually doing pretty well though he is battling daily seizures which started not too long after Dana’s passing. Before we turn the slide and I get into some discussion about our experience in the Phase I trial, I would like to tell you all how important we feel this trial is for all of the kids and for the adults as well with NPC. We have dreamed of an approved therapy as a collection of families. The NIH has provided amazing support in this effort and the US FDA has listened to our pleas. Those of you who have been to our fundraisers have heard me say that I like to describe this community as an extended family, a committed family, and I hope with this trial, we will continue to show that to everyone.
- 45. Potential Impact on Hearing Anunsettlingrisk;decisiontoacceptornot 45 • March 2015: our son, Andrew, received 1st dose at 900mg • Andrew had a significant loss of hearing in the mid-conversational range • For Andrew’s April visit, we’re thrilled his hearing has come back nicely • Hearing impact was a Grade 1 loss • Realizing NPC is already taking Andrew’s hearing, we bought hearing aids for Andrew • Andrew is hearing things he has not heard in years • Hearing aids are usually covered by insurance for children • We worried non-stop about risking Andrew’s hearing • Now feel comfortable that the loss can be addressed • Excited to see Andrew more talkative and walking better • It’s a very personal decision, but it is important to remember that NPC is impacting hearing anyway, and it’s the other, devastating, mental and physical deterioration we hope to dramatically show down
- 46. 46 The first thing that I thought I would address is the issue that we all worry the most about which is the hearing. As you know, the mice and the cats are completely deaf and irreparably deaf. And so, that became much more of a concern particularly as we started approaching higher doses this past fall. I'll tell you a little bit about our experience. Our son Andrew has been receiving an increasing amount of cyclodextrin for 17 months now. Last month in March, he received 900mg for the first time. As was expected, Andrew had a significant hearing loss mostly in the midrange sound. If you have learned about this at this point, it is very important for conversation. In the time between our April and March visits, we went ahead and got Andrew hearing aids. Last week we returned to the NIH for the second dose of 900mg. We learned that Andrew’s hearing had actually recovered pretty nicely. When we left after the first dose of 900mg, his hearing was graded as a grade 3 loss but by last week it had recovered to a grade 1. That was a great bit of news.
- 47. 47 Now, I mentioned that we had already gotten Andrew hearing aids. We were actually inspired by Tonya Kains' story, which she told to my wife Andrea in March. The Kains' had gotten their daughter Julia, who is about Andrew’s age and a wonderful young lady, hearing aids. Tonya was telling us the story that Julia got up one morning, put in her hearing aids and gleefully came downstairs to tell her mother she heard the birds chirping outside for the first time. That was when we realised that we had sort of been depriving Andrew of some of the pleasures of the world. He had already had some fairly significant high range hearing loss and that was something that we had just sort of thought that he could get by without the hearing aids. I have now gotten him hearing aids. I have to tell you his hearing is almost perfect at this point. These hearing aids are now so sophisticated they can literally tailor them to the loss that he has. We were there this morning adjusting them for the increased recovery in his hearing over the last month. They actually tuned in two programs so that when we go back we can push this little button three times and it will increase the benefit for him for a period of time after he gets the next dose. When it gets a little better, we can press the button three more times and it will go back to the original setting. It is absolutely amazing what you can do with the hearing aids now. The good news for families with children is that it is usually paid for by insurance whereas, not for adults.
- 48. 48 I can't really express the fears that we had going into that 900mg LP. It is very easy to take a logical approach and say, I'm giving this medicine, it is going to improve his life, and it is going to give him a longer life span; but, the moment before you give your child something that may rob their hearing the next day, your heart is going to stop to beat. But having taken the step, having had a much better result than we thought was likely, we really feel some relief at this point about cyclodextrin. We’re not concerned about going to 1200mg any longer. I think that with the hearing aids, it is just amazing what we have been able to do for Andrew. And of course, we are hopeful that the cyclodextrin is going to have great benefit for his overall quality of life. We are actually encouraged over this past year. He is talking more, he's walking better. He was at physical therapy this morning and his physical therapist is very pleased with his increased balance, strength in his legs, and his gait is better. It is certainly looking positive and I think everyone has the same kind of hopes for cyclodextrin.
- 49. 49 But needless to say, this is a very personal decision that everyone has to make for their family, for their child, and for themselves about whether to take the cyclodextrin and risk what is still an undefined potential risk with the hearing. I think it is important to note though that, as I said, Andrew already had some significant high frequency loss and the NPC is taking the children’s hearing anyway; so, when you put that up against the potential benefits, I think the goal is to dramatically slow down the rest of the horrors of this disease because we are losing the hearing anyway.
- 50. Lesser Challenges Thingsthatarenotasbadastheyseem 50 Having reconciled what our family sees as the greatest challenge, we face the lesser challenges • Lumbar punctures • Andrew has had almost 25+ LPs since 2006, and only 1 with some post-LP nausea. The last 3 were with just local anesthesia, and he did great. • To move quickly with the treatments, which is so crucial for all of these children, LP seems the best way to go. Ommaya reservoir was a clever idea, but it cost us all critical months of delay. • Frequent travel to the site • A necessary sacrifice to have the proper control, but hopefully enough approved locations to make it as convenient as possible. The locations have to be facilities that can meet the standards for potential approval. • Control arm in the experiment – Promised my family I’d always work to avoid another “Zavesca Situation” • During the controlled trial portion, there is a chance that your child will not get the drug for the first year • After that everyone will be enrolled in an open label extension where they will get the treatment
- 51. 51 I would like to address some of the other issues that I see as less problematic. They are somewhat obviously traumatic in our minds, but I think having faced down the biggest challenge of the hearing, we're not quite as concerned about these. That is the lumbar punctures which no one particularly likes; but, if we want to move this trial forward quickly, this is the best way to go. I will tell you that Andrew has had over 20, maybe 25 lumbar punctures since 2006. He was one of the first children enrolled under the natural history study. In all that time, he has only had one lumbar puncture that resulted in some post LP nausea. He didn’t even have a serious headache from it. The last four LPs with the cyclodextrin were done with local anaesthesia and he did really, really well. To move forward with the treatments which are so critical for all these kids, LP seems to be the best way to go.
- 52. 52 I was very excited about the concept of the Ommaya resevoir when it was first presented, but obviously when we developed some difficulties with it, it cost us critical months of delay. Anything that we would do along those lines again would likely lead to further delay and deterioration of children unnecessarily. Anytime you are in a drug trial, you just don't do this at your local hospital. There are going to have to be limited number of sites. There will be frequent travel. It is certainly going to be easier with multiple sites. It will be easier than going to the NIH once a month, but we are not going to be able to do this everywhere and we are going to have lots of number of sites and that will hopefully help us create the right environment to get the approval. I am told that there will be a number of sites and they are working very hard to make this as convenient for everyone as possible.
- 53. 53 Ben and Marc talked about the control arm in a trial, which again we all wish was unnecessary in any drug trial. I have to say that I promised my family after the Zavesca fiasco, that I would always work for this community to avoid that again. I was one of the three parents that spoke before an FDA panel that was charged with recommending one way or the other whether to approve Zavesca or not. I will tell you that listening to the onslaught of complaints about Zavesca from the FDA was devastating. I was just completely overrun with the complaints. The sad thing was we knew it had helped the children, but we couldn’t prove it. Here we are with a situation in this country (US) where about half the kids can get Zavesca and half the kids can't. That is a shame and that is something we have to avoid for all of our potential therapies or hopefully approved therapies in the future. During the controlled trial, there is a chance your child will not be getting the drug for the first year; but, there is a commitment that after you have been in the trial for 12 months, you are going to be enrolled in open label extension where you will actually get the treatment as long as it takes to get approval. Hopefully, we get the approval.
- 54. Our Hope • Quality-of-life and longevity that we have seen in mice and in cats is the strongest that we have seen with any drug • This drug has been used safely in several other drugs as a carrier – has a large safety record behind its use • Controlled experiment • Only a clinical trial will demonstrate if we can see the kind of success in children with quality-of-life and longevity that we have seen in cats • We cannot have ANY more treatments which some of the children can get and other children cannot. That takes FDA approval • The Zavesca approval process underscores this – the panel even accepted that there was a “suggestion of benefit”, there was plenty of personal evidence but not enough scientific evidence for approval • Controlled clinical trial will help get that evidence and accelerate the approval process 54
- 55. 55 Let’s look at the positive side of this. What are our hopes as parents, as patients, and as the doctors of patients? We’ve hoped forever for an approved therapy. The quality of life and longevity that we have seen in the mice and in the cats is the strongest that we have seen from any drug ever. cyclodextrin has been used safely to deliver other medicines. It has a large, long safety record. We are conducting a controlled experiment approved by the FDA and the EU and only a clinical trial can demonstrate if we are going to see the kind of success in children that we have seen for example in the cats. We can't have any more treatments where some of the children can get the medicine and some of the children cannot. That takes FDA approval. The Zavesca approval process underscores this. The panel even accepted that there was a suggestion of benefit. That afternoon, we convinced 10 out of 13 panelists with our hearts to recommend the approval of Zavesca. A controlled clinical trial will help get the evidence and accelerate the approval process.
- 56. 56 A couple of closing points. I think it is important to remember as Marc said, cyclodextrin presents the greatest possible benefit we've ever seen for the kids, but it is not a cure. This is just a first step; but, clearly an important step in what we hope will be an approved combination therapy, a drug cocktail if you will. Hopefully by the time this trial is over, we will have something else, maybe more than one item ready to roll into another trial - something that we can build on. We have developed a lot of goodwill with the NIH, and with the FDA. I hope that we can follow through on the earlier enthusiasm we all showed and make this trial a success. Thank you.
- 57. How Do We Get VTS-270 Approved? • Clinical trials are experiments to answer several key questions: • Does the drug work? • Which aspects of the disease does the drug treat? • What are the risks and side effects? • What is the dose where the drug works best (best efficacy) and has the lowest risks/side-effects (best safety) • Current Phase I will provide initial answers for some of these questions; provides the basis for Phase II/III clinical trials • Vtesse will conduct a combined Phase II and Phase III clinical trial that will seek to provide definitive answers to these questions • Regulatory bodies will review our data to see if we have established safety and effectiveness of the drug to support approval 57
- 58. CONFIDENTIAL & PROPRIETARY VTS-270: PATH FORWARD Ben Machielse, Drs. Vtesse President & CEO 58
- 59. 59 Thank you Phil. Let me talk a little bit on how do we plan to get VTS-270 approved. As Marc said, clinical trials are experiments and they are designed to answer several key questions. First of all, does the drug work? Second, we plan to actually set up a study such that hopefully at the end of the study we understand a greater aspect of the disease, if the drug works. Hopefully we will find out about certain key aspects - that cyclodextrin has a positive effect on NPC but in other aspects, it probably will not. By doing the study, we may be able to provide a benchmark and understand where cyclodextrin works and where it does not work. That would open up the way for future development of drugs which cover the aspects of treatment where cyclodextrin doesn’t work. Also very important is will we understand the risks and the benefits and the side effects of the drug? We have talked about ototoxicity, but we need to make sure that there are no other side effects and risks involved with administering this drug.
- 60. 60 Also very importantly, what is the best dose? What generates the best efficacy and has the lowest risk side effects? We need to carefully balance that and we plan on doing that in this study and I will explain that somewhat later. Fortunately, the current Phase I study as conducted by NIH will provide initial answers for some of these questions and it will provide a basis to actually build clinical protocol for the Phase II/III trial. We focus on a single trial which hopefully provides definitive answers and can help us to seek approval of this drug. After completion of the clinical trial we will work rapidly to submit the documentation to regulatory agencies in both the US and Europe and other territories to seek approval.
- 61. Our Current Thinking for a Pivotal Clinical Trial • Global, 50 patient, multi-site study will be launched no later than second half of 2015 • Controlled study (treatment and control arms) in 2 stages: • 1st stage to select best dose • 2nd stage patients get the best dose • Intrathecal, bi-weekly administration of VTS-270 with 1 year duration • Followed by an open label extension until the time of approval • Both the treatment and control arms will get the drug 61
- 62. 62 I would like to talk to you a little bit about our current thinking for our pivotal clinical trial. I just want to preface this: that we cannot disclose everything yet because we are still in active discussions with the regulators, but what we can disclose is the following: we plan on a global clinical trial which means that we will design such as capture the patients as quickly and as efficiently as possible. We plan on possibly somewhere between 15 and 20 sites worldwide to actually conduct this study. It will be a multi-site study in multiple territories, and we also will provide support to travel to the sites. We understand that even with 20 sites there still will be travel involved and we will do our utmost best to make it as easy and as convenient as possible for you. We plan on launching the site no later than the second half of 2015 and I'm optimistic that we actually can start this trial for you in Q3 of 2015. We also will capture a lot of other data. We will evaluate what biomarkers could be indicative of efficacy and those kind of things, but of course the most important thing is that we look at clinical phenomena which could be indicative of efficacy of the drug.
- 63. 63 We plan on a controlled study and as Phil said, a certain number of patients will not receive the drug for a year. We are not thinking about one to one randomization, but we are currently thinking about a two to one randomization, because we are very sensitive about the fact that certain patients will not receive the drug for the first year. It also will be a blinded trial, and as you may hear I'm not using the word ‘placebo’ anywhere here. We are doing a controlled trial where we basically will simulate everything for each patient in the trial. Patients in the control side of the study will not receive the lumbar puncture in the lower part of the back. We are also evaluating what we call the implementation of a rescue option. If there is unexpected decline in some of the patients, we are evaluating if it would be possible to implement an option where those patients are lifted out of the trial and put on drug. That is what we are evaluating, this is not yet a hard commitment, but we are working on it.
- 64. 64 The study consists of two stages. The first stage is to actually select the best dose. We will assess safety tolerability and side effects of the doses so that we actually have an indication that we can enroll the remainder of the patients in Phase II. As I said before, it will be an intrathecally administered product. We will administer it every two weeks for the duration of one year. I also want to emphasize that we looked at multiple dimensions of this. We are not scoring people only on one particular aspect; but, we have designed the study such that we will evaluate in what aspects patients will stabilize or maybe even improve. After a patient is enrolled for one year, each and every patient in the clinical trial will be allowed to participate in the open label extension study until the time of approval. That means people who were treated and also people who were in the control arm will receive the drug after that first year of study until the time the drug is approved. We try to be very sensitive to your needs. We do believe that a controlled study is required given the fact that the disease progression is relatively unpredictable and we must be able to accurately measure any effect of cyclodextrin. That concludes our presentation and I would now like to answer some of the questions we have received during this session.
- 65. Discussion • Questions from the community 65
- 66. 66 We did receive many questions. As we indicated earlier we cannot answer all of them here. There are quite a few questions around the lumbar puncture. I will try to do so and you know fortunately we have people here on the panel with significant experience with these repeat lumbar punctures and two of those are here, Liz Berry-Kravis and Denny Porter. I would like to ask their opinion about their repeat administration of lumbar puncture. How patients perceive it and what you consider be the risk of that? Maybe Liz you can start and comment on this. Okay. Hi, thank you for letting me participate in this webinar. We have an iIND in which we have three patients enrolled and two of our patients have been enrolled for about 16 months now. We have been doing LPs every two weeks on these patients and in general this is really very well tolerated. The process itself is really not that difficult. It is mostly a matter if the person doesn’t understand what is going on, they can show that they can be still for it and not move during the process. In many cases doing an LP on a child is almost easier than drawing blood because the spaces between the bones and the spine are quite wide and you don't have problems with arthritis and it is really pretty easy to do.
- 67. 67 What we do when our patients come in is we have Lidocaine that we put on the back. It is a cream and kind of numbs them up and then when we do the spinal tap, we inject some additional Lidocaine, but they don't feel the needle so much because we have already numbed up the back with the cream and then just put the needle into the back, get fluid out and then do the infusion. As long as the patient is lying still, they are actually pretty comfortable. Our patients joke around with us, talk with us and we talk about movies during the procedure. Our patients have been good afterwards. They haven’t had any problems. We did have some problems with post spinal tap headaches and a little bit of vomiting initially; but, we switched to a special kind of needle called the Whittaker needle which doesn’t cause as much damage to the covering of the spinal cord when it goes in, and we haven't had any problems really with those kinds of side effects afterwards. We do the procedure in the outpatient clinic with our patients. It takes about 15 to 20 minutes and our patients are in and out. They come in the morning and they are out by early afternoon. It takes some getting used to but once you get used to it, it is kind of an aggravation but it is not really a big difficult thing. Okay thank you Liz. Denny would you be able to share your perspective?
- 68. 68 Yes. Lumbar punctures are one of the items when we go through the consent that we always end up focusing on because almost always the parents or guardians are concerned about it. It really is a simple procedure and Liz described it well. Now that is coming from the perspective of a paediatrician, not a parent. It tends to be scarier in concept than it is in actuality and what the risks are. The risks are really the post LP headache, plus or minus some vomiting or nausea associated with it. We've done LPs in the Natural History trial and now in the cyclodextrin trial. We have done hundreds in NPC kids and young adults. Phil talked earlier about his son’s experience and that’s pretty typical. One episode of nausea with about 25 LPs. Our numbers are somewhere around that 5% mark. The headaches or the nausea tend to be self-limited and relatively short in duration. Very rarely do they last beyond the day. It is something that, yes it is an adverse event, it will happen if we do enough of these, but it is a very minor problem.
- 69. 69 The kids tolerate the procedure very well. At least half of our kids do it at the bedside as Liz described. They are very used to it and the teenagers will sleep through it. The younger kids we do have to sedate, but again, it is a quick, relatively easy procedure with very little actual risks that occur. It’s tolerated very well. Kids tend to let you know when they don't like something. We have some kids that obviously get nervous, but they are nervous in general. But we don't have kids objecting to this being done and as every parent knows if your kid doesn’t want something they tend to let you know. Thank you Denny. Marc anything to add?
- 70. 70 No I would support exactly what my colleagues said. I would just emphasize the fact that I think the general public has a misunderstanding of this procedure, and as I said in the earlier webinar, it is often done in emergency situations in less than ideal circumstances. Sometimes by people who are not very experienced at doing it. That is very different from the situation in a clinical trial or in a diagnostic setting where you have operators who do this all the time. In our institution we have a service actually run by nurses who spend all day doing spinal taps. They do thousands of them and as Denny pointed out the rate of headaches afterwards is about 5% and otherwise, we really don't see major problems with this. I have probably seen most children who have had a chemotherapy for leukaemia and these children have the intrathecal chemotherapy and then jump up off the bed and then go and play. They get used to it, and I would agree it is really not that much worse and in some ways, easier than drawing blood from some children.
- 71. 71 Thank you Marc. I would like to add that when we evaluated the development of this drug, we also evaluated if it made sense to first develop the alternative delivery device and then start a trial. But we came to realize that it could take a year to two years to do this and we really believed that getting an answer on cyclodextrin quickly is absolutely essential. I can also assure you that Allan Darling, our head of technical operations is working actively on evolving this device. We are currently in the process of identifying those devices and doing the initial evaluation. I cannot guarantee when it will be done but it is one of our priorities, just wanted to add that.
- 72. 72 Many of the questions are around a controlled trial. Why is a controlled trial important? We believe that a controlled trial will give us the objective evidence that the drug works and it shows us that it is effective. This is a disease which has highly variable disease progression and finding out what the drug is actually doing without the control is very difficult because you do not know what your reference point is. We believe that it is very important to make the most accurate assessment possible about the efficacy of cyclodextrin. In turn, this improves the probability of success which is of long term interest to the NPC community. I do believe that running a controlled trial will also allow you to shorten the duration of the trial because you have an immediate direct comparator. In addition, it will give us the opportunity to identify specific effects of the drug. We talked about that. This will be important for patients and caregivers to exactly understand what the drug will do and what it will not do.
- 73. 73 Because it is important to develop the best treatment regiment possible for the children who have NPC, we believe that it is important to do a controlled trial. We also understand that there is concern about the controlled trial; but, we think that from a long-term perspective it is the best thing to do. Again, the study itself will take a year. We will administer the drug every two weeks and we are also considering the rescue option to minimize the risk to the patients who are not treated, and we have the open label extension study. We are sensitive to this concern but we are also actually asking you to take a long-term view on how to develop this drug and make it available to all children.
- 74. 74 There is one last question we can take and I would like to answer that and that is the question on the travel, and the burden of the travel and support. Our strategy is to actually be very opportunistic in defining where sites will be located. It will be driven by where the patients actually are. It will be determined by the quality of what else we can find in the vicinity, and we will do our outmost best to make sure that everybody that’s interested in the study and qualifies, can participate in the study with our support and with reasonable access to the sites. Of course we are currently working with our CRO. We actually tried to map out where the patients are, and that will drive to where we will locate the study sites. Having said that, I think we have come to the end of the webinar. I would like to thank you for your time. I hope that you realize that this is the first webinar, of hopefully many to come. We would love to hear from you. I promise you that as soon as we have more information, we will schedule the next webinar. Thanks again for all your time. Have a good evening and hope to speak with you soon.