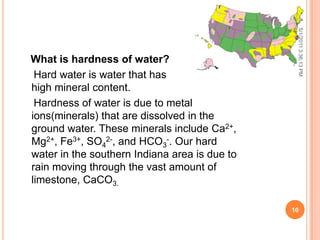
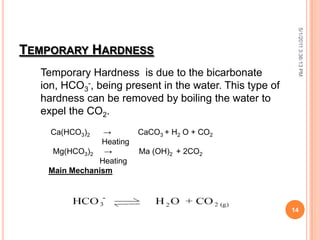
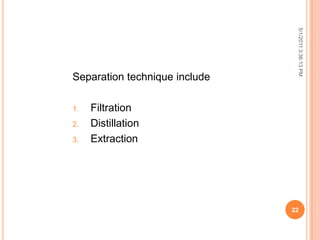
The document discusses water analysis and quality. It covers various topics related to water including hardness, dissolved and suspended solids, and separation techniques. Specifically, it defines hardness and the different types, explains why dissolved and suspended solids impact water quality, and outlines common separation methods like filtration, distillation, and extraction.





















![REFERENCES
Map from Morton Salt at
http://www.mortonsalt.com/soft/sofisoft.htm
^ a b c World Health Organization Hardness in
Drinking-Water, 2003
^ a b Hermann Weingärtner, "Water" in Ullmann's
Encyclopedia of Industrial Chemistry,
2006[december], Wiley–VCH,
Weinheim.doi:10.1002/14356007.a28_001
http://www.glendalewaterandpower.com/residents/
water_hardnes
http://www.mrwa.com/OPWater%20and%20Impuriti
es.pdf
5/1/20113:36:13PM
26](https://image.slidesharecdn.com/wateranalysis-130526205208-phpapp01/85/Water-analysis-26-320.jpg)