The cement manufacturing process involves several steps:
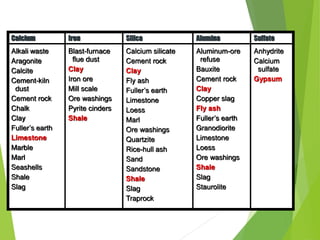
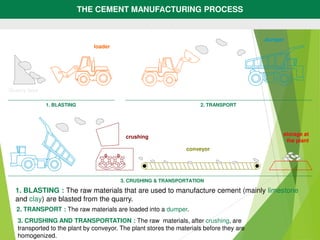
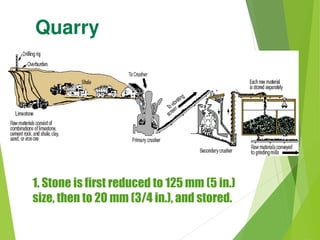
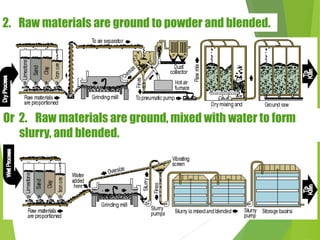
1) Raw materials like limestone and clay are crushed and blended in precise proportions.
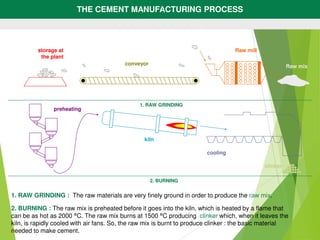
2) The raw mix is heated in a kiln at high temperatures up to 1500°C, which causes chemical reactions that produce clinker.
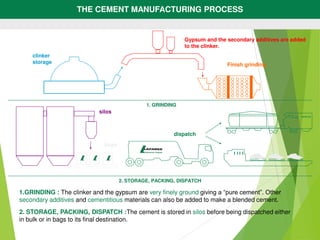
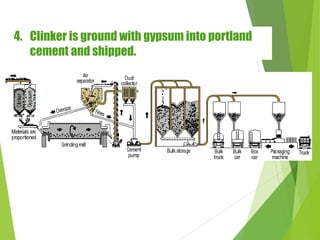
3) The clinker is cooled, ground with gypsum, and packaged for distribution.