The document summarizes key concepts in thermodynamics including:
- The second law of thermodynamics which states that heat cannot spontaneously flow from a colder body to a hotter body.
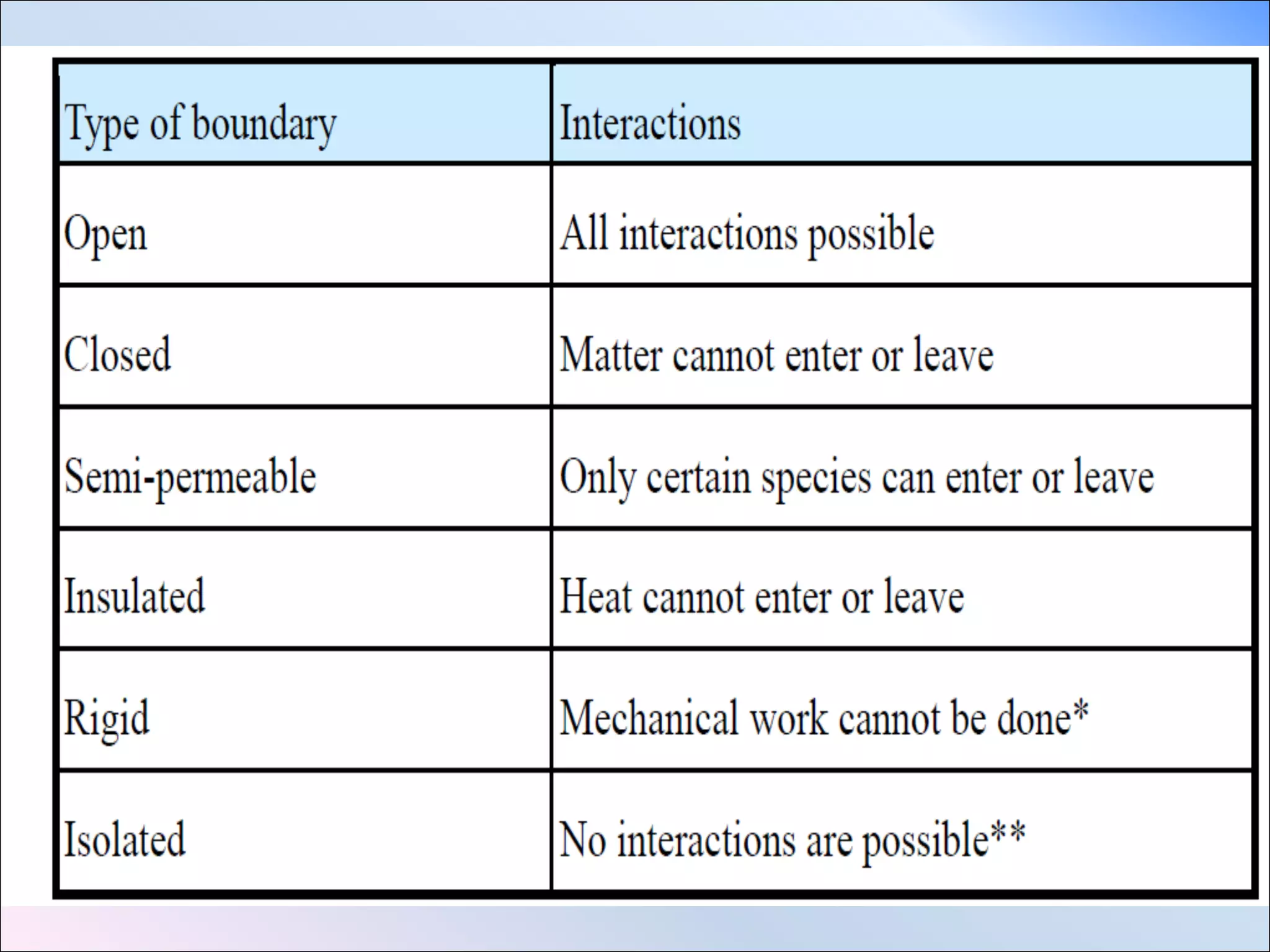
- Closed, open, and isolated systems. A closed system does not allow matter to enter or leave, an open system does, and an isolated system exchanges neither energy nor matter.
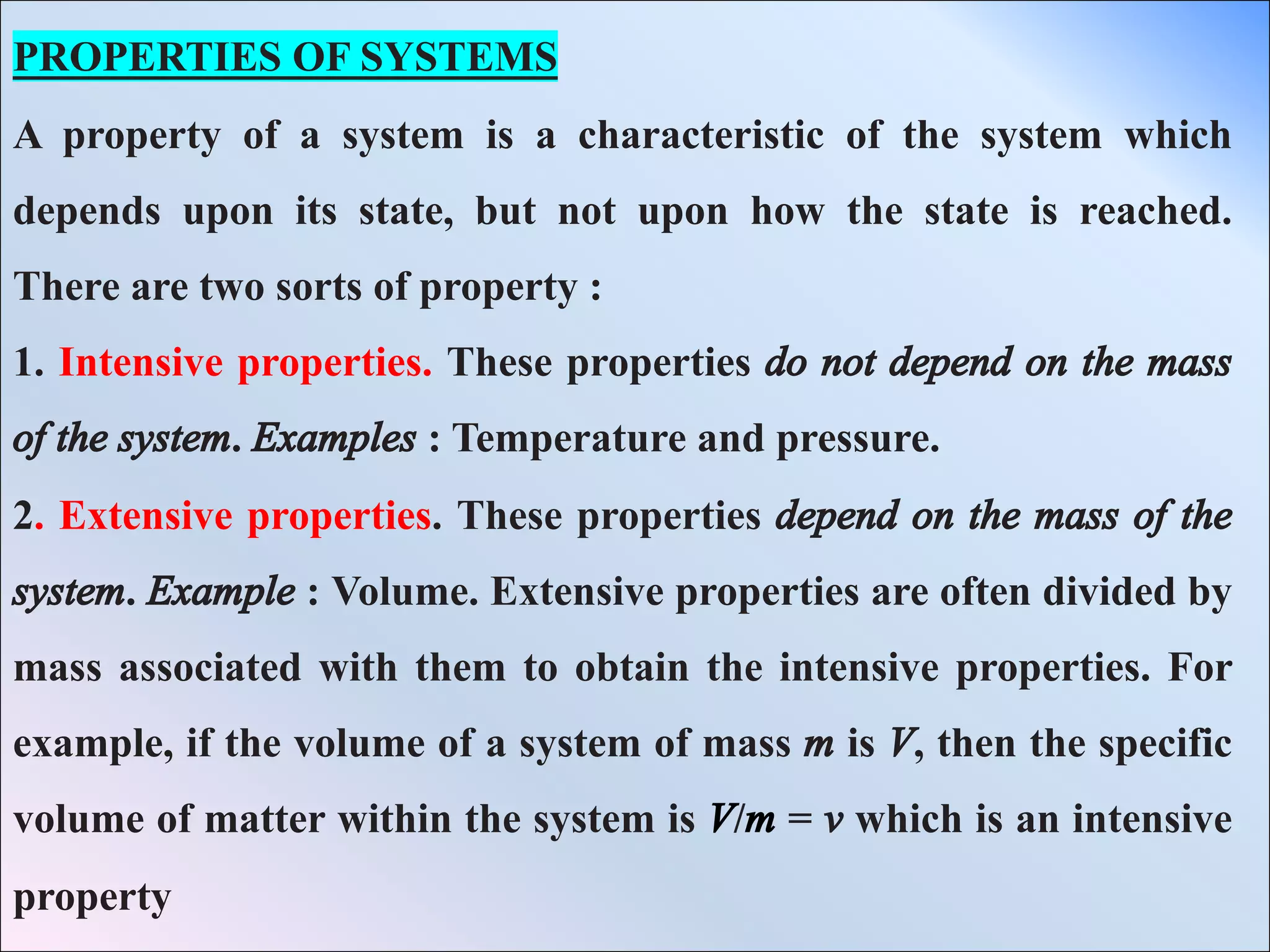
- Properties of systems including intensive properties which do not depend on mass like temperature and pressure, and extensive properties which do depend on mass like volume.
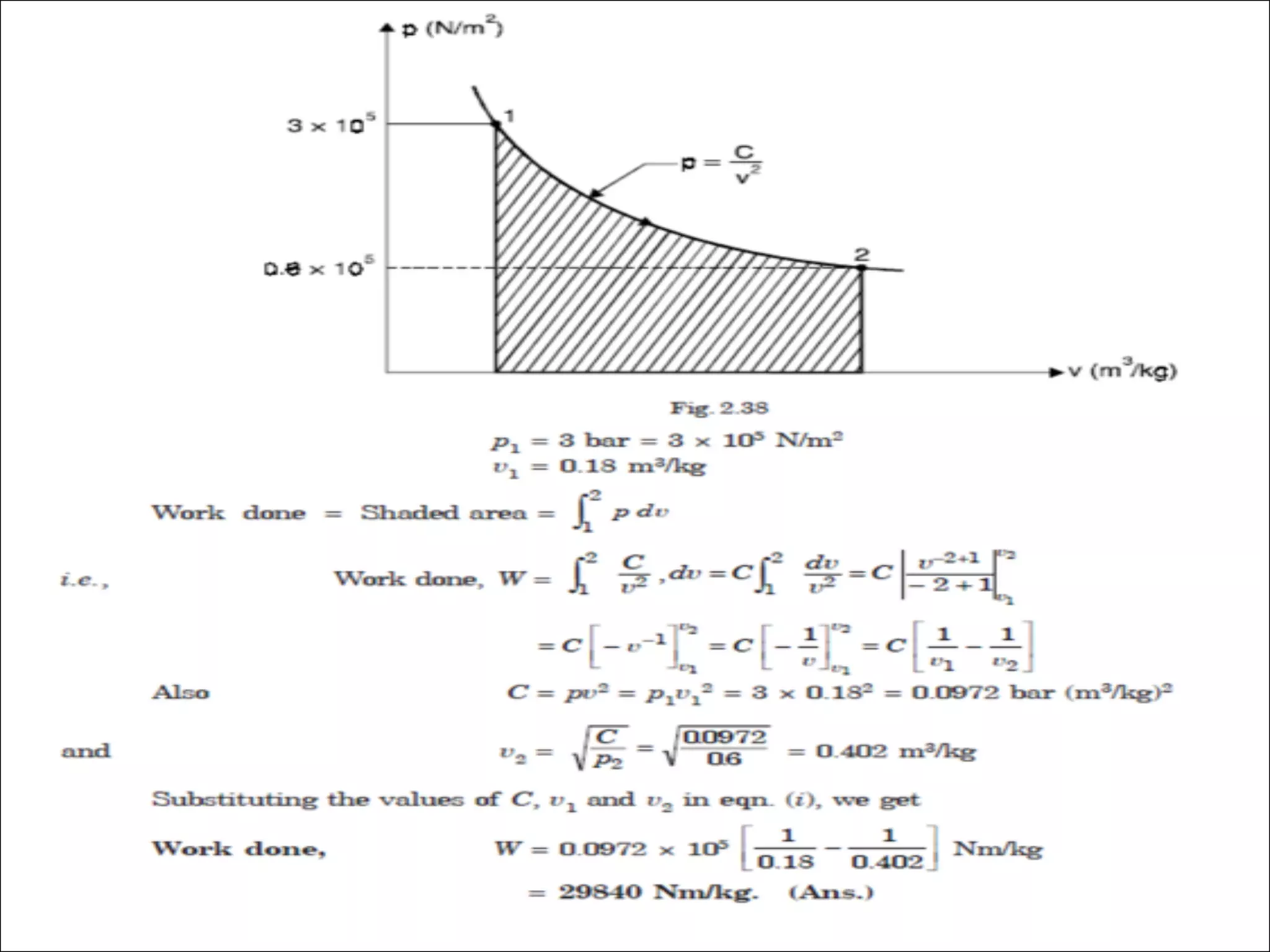
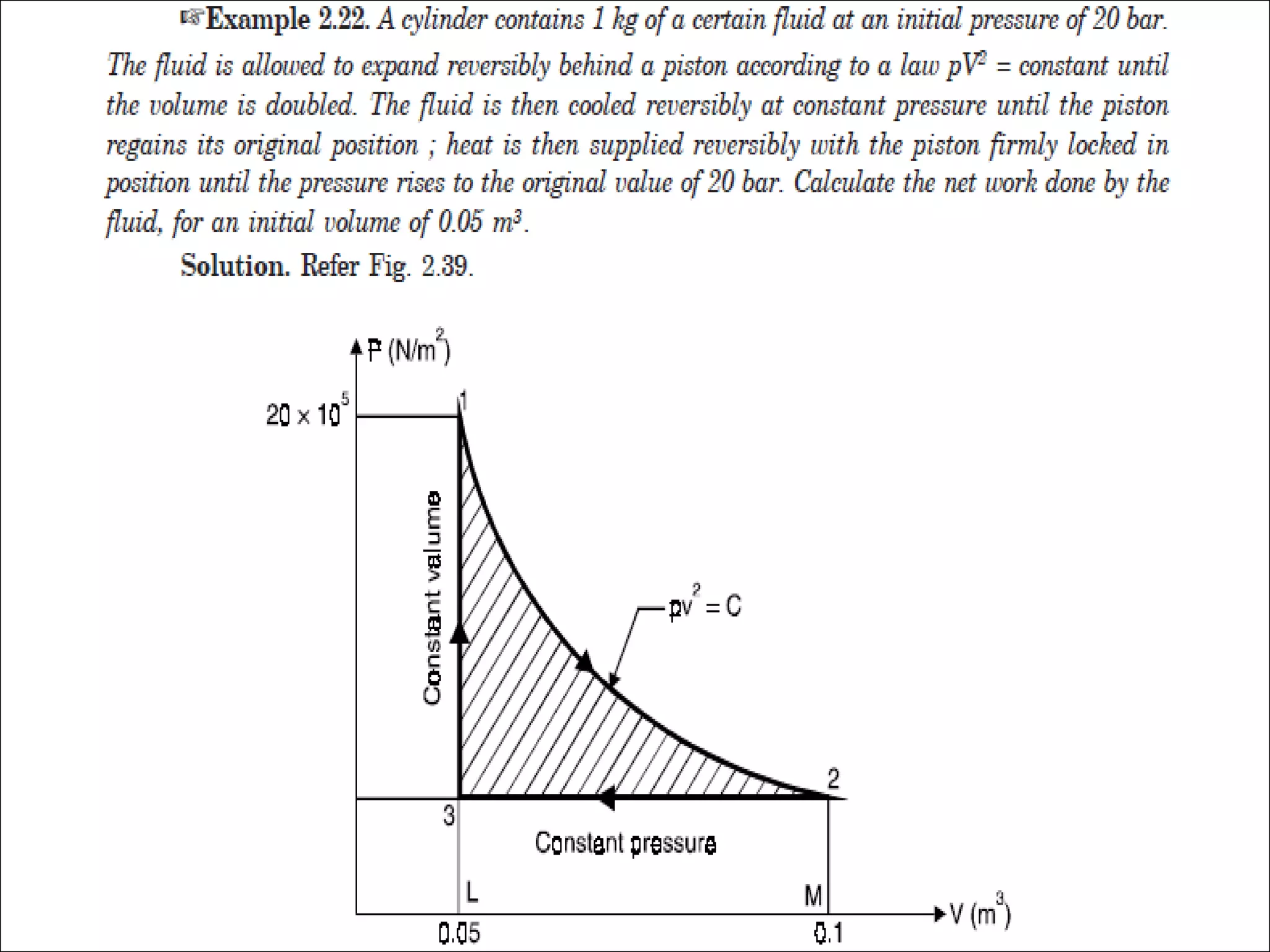
- A cycle occurs when a process returns a system to its initial state. An ideal gas follows Boyle's law that pressure and volume are inversely proportional.