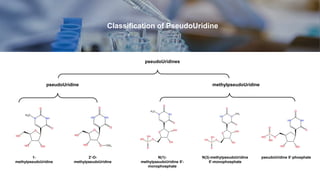
The document provides a detailed classification of pseudouridine and related molecular entities, including definitions and formulas for various compounds such as c-glycosyl pyrimidines and methylpseudouridine derivatives. It outlines the structural distinctions and functional relationships of these compounds in the context of organic chemistry. Additionally, the document includes contact information and organizational details related to the classification of these chemical entities.