Embed presentation
Download to read offline

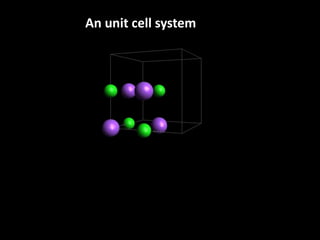
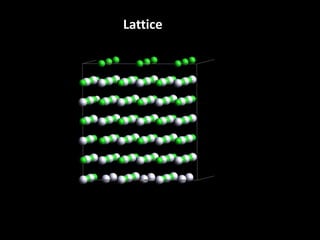
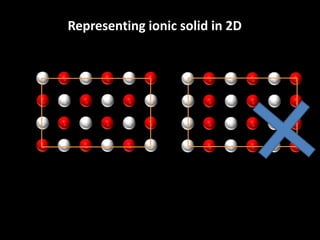
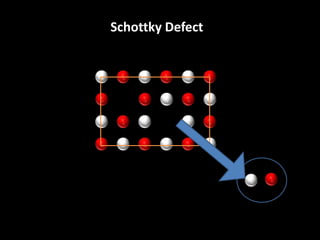
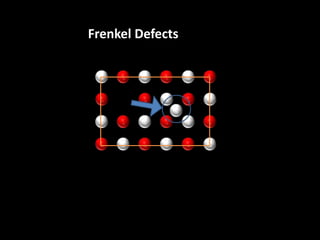

This document discusses different types of defects that can occur in solid materials, including Schottky and Frenkel defects. It defines lattice structures and unit cells used to represent ionic solids. Equations are provided that calculate the number of Schottky defects and Frenkel defects in a material based on parameters like the number of sites, work required to form the defects, temperature, and gas constant.






