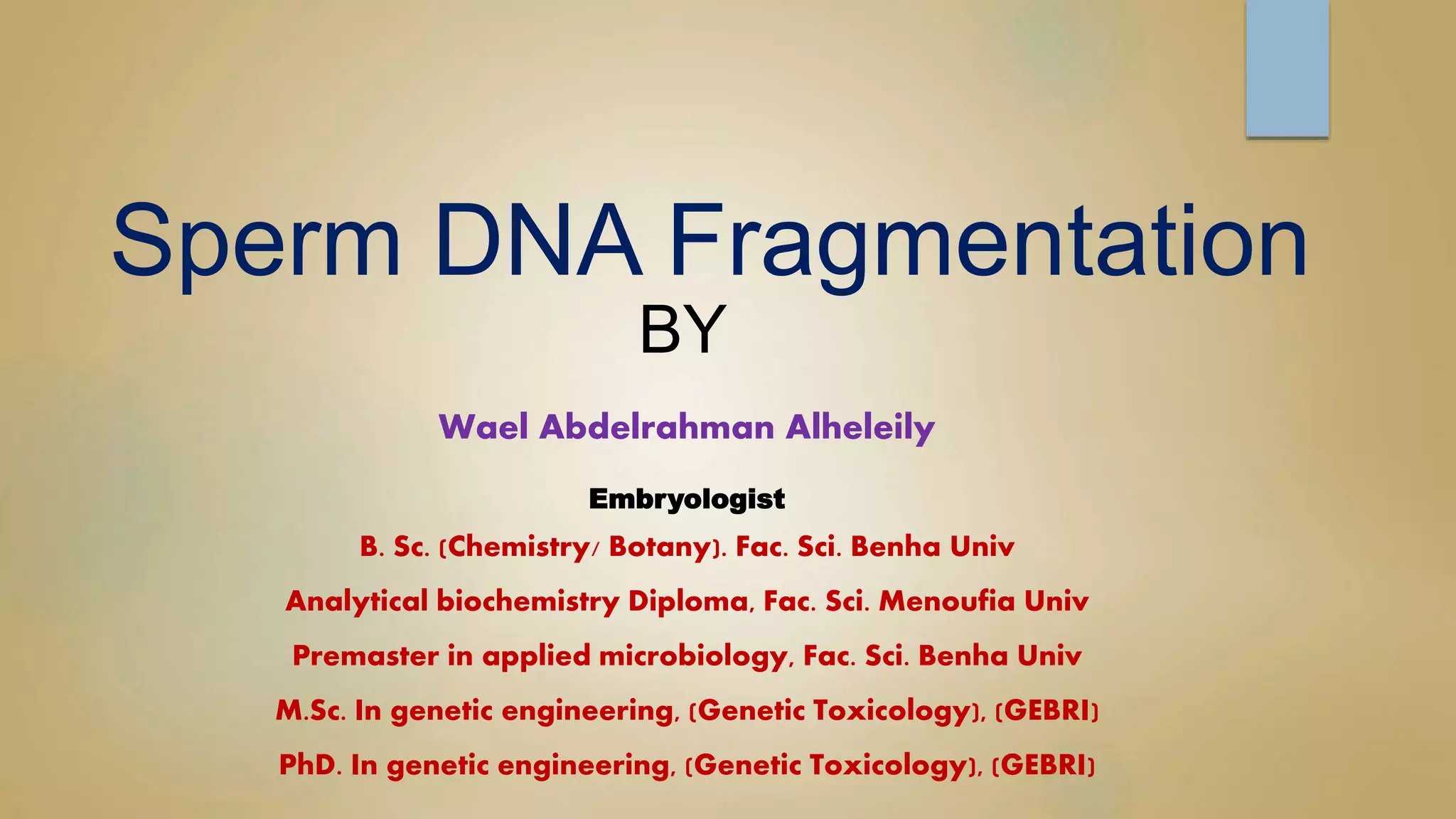
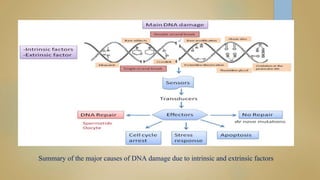
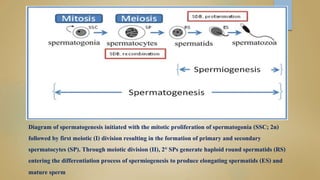
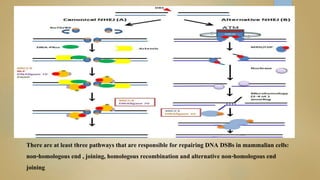
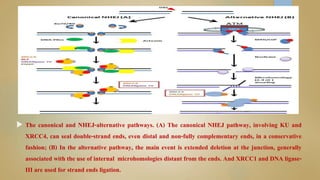
Sperm DNA fragmentation can negatively impact fertility and pregnancy outcomes. DNA becomes vulnerable during spermatogenesis when protamine replaces histones in chromatin compaction. Tests can evaluate sperm DNA fragmentation levels, which are correlated with lower fertilization and implantation rates. Factors like oxidative stress, temperature, and infections can intrinsically or extrinsically damage sperm DNA. Repair mechanisms attempt to resolve DNA double-strand breaks, but extensive unrepaired damage may be incompatible with embryo development. Treatments aim to address underlying causes of DNA fragmentation or use testicular sperm or ICSI to bypass ejaculated sperm issues.