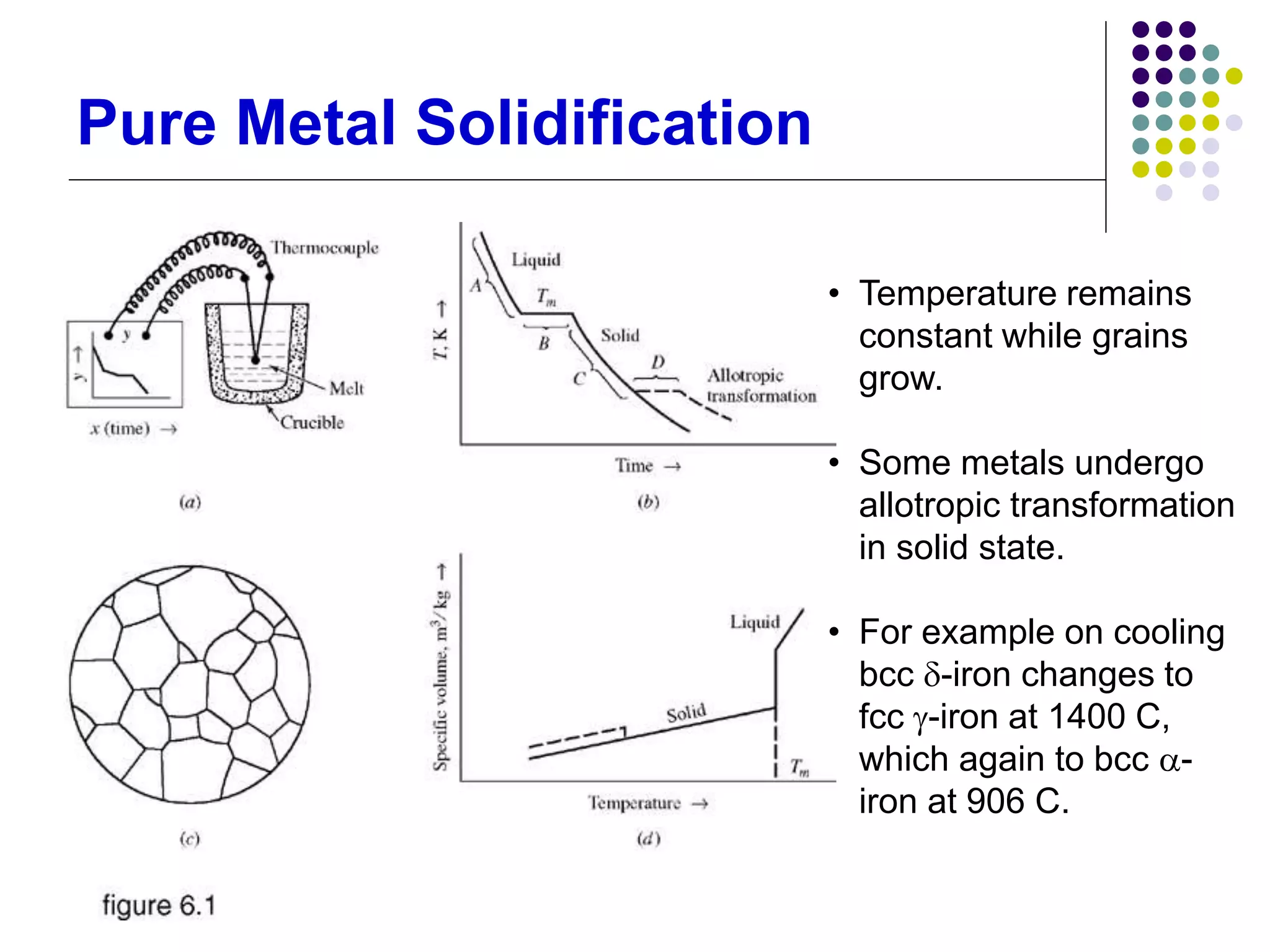
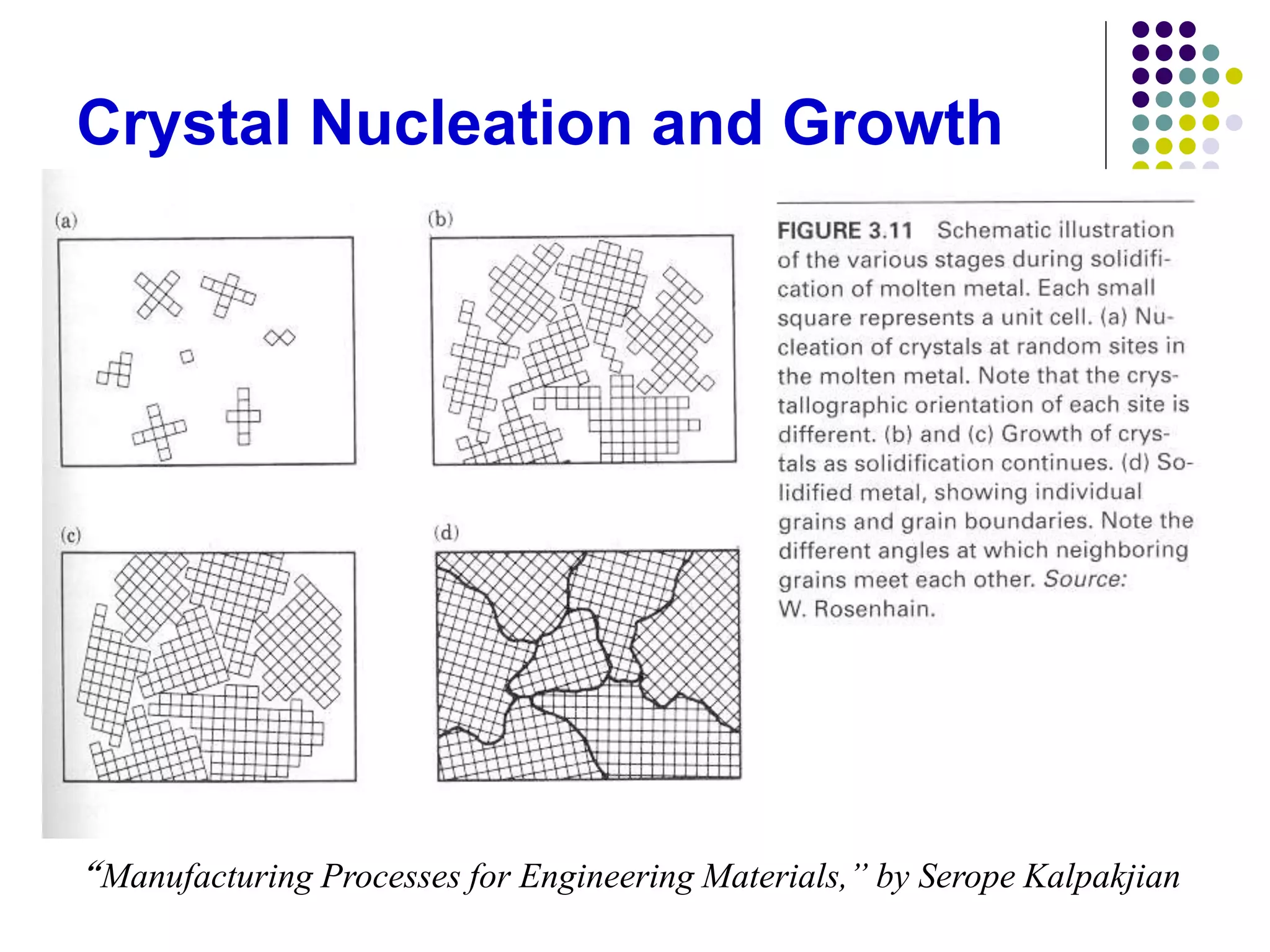
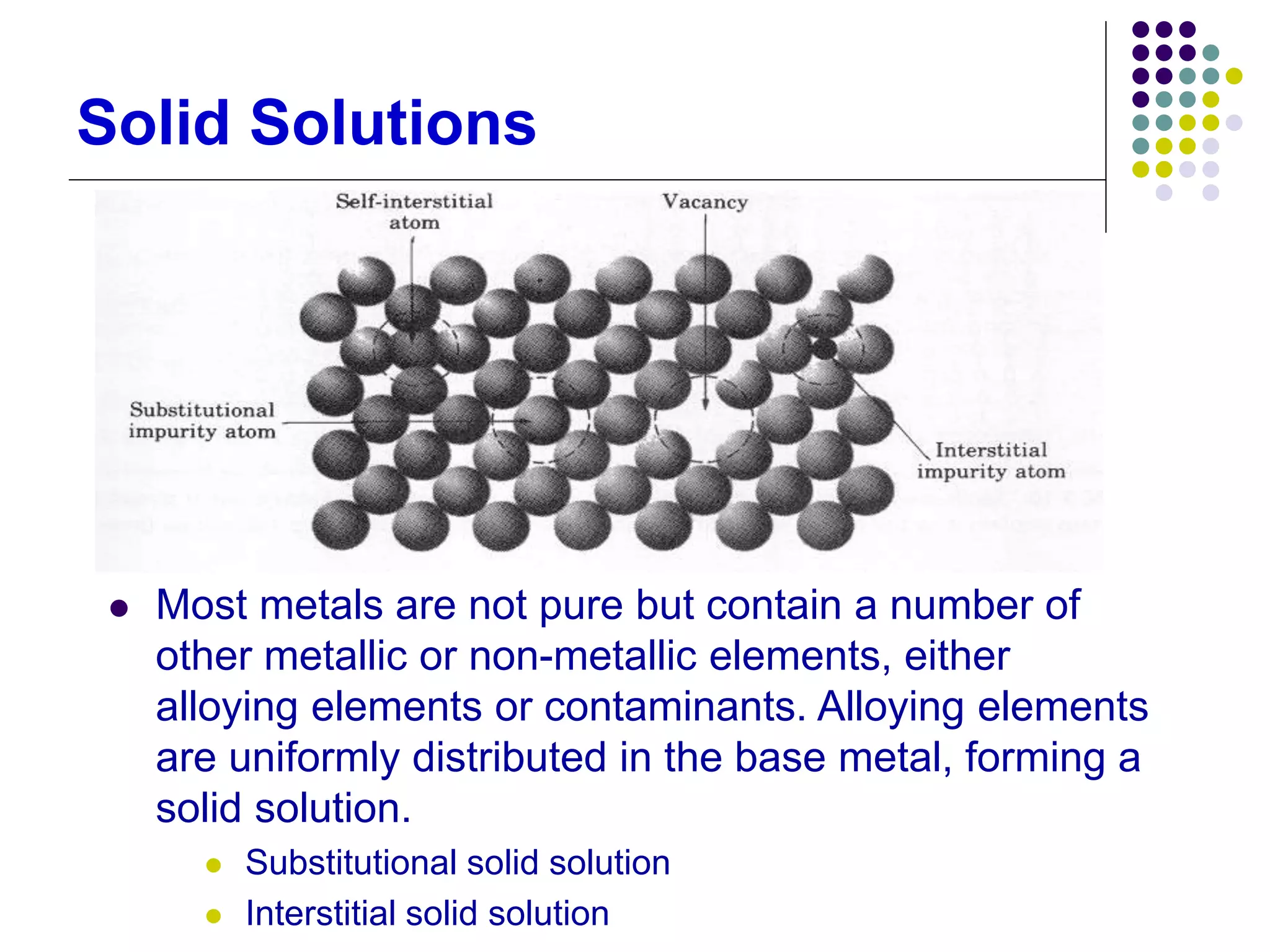
This document discusses solidification processes and how they affect crystal structure and material properties. It covers topics like nucleation and grain growth during solidification, different crystal structures, the effects of imperfections and grain size, phase diagrams, and how heat treatments can modify material properties by changing the crystal structure.