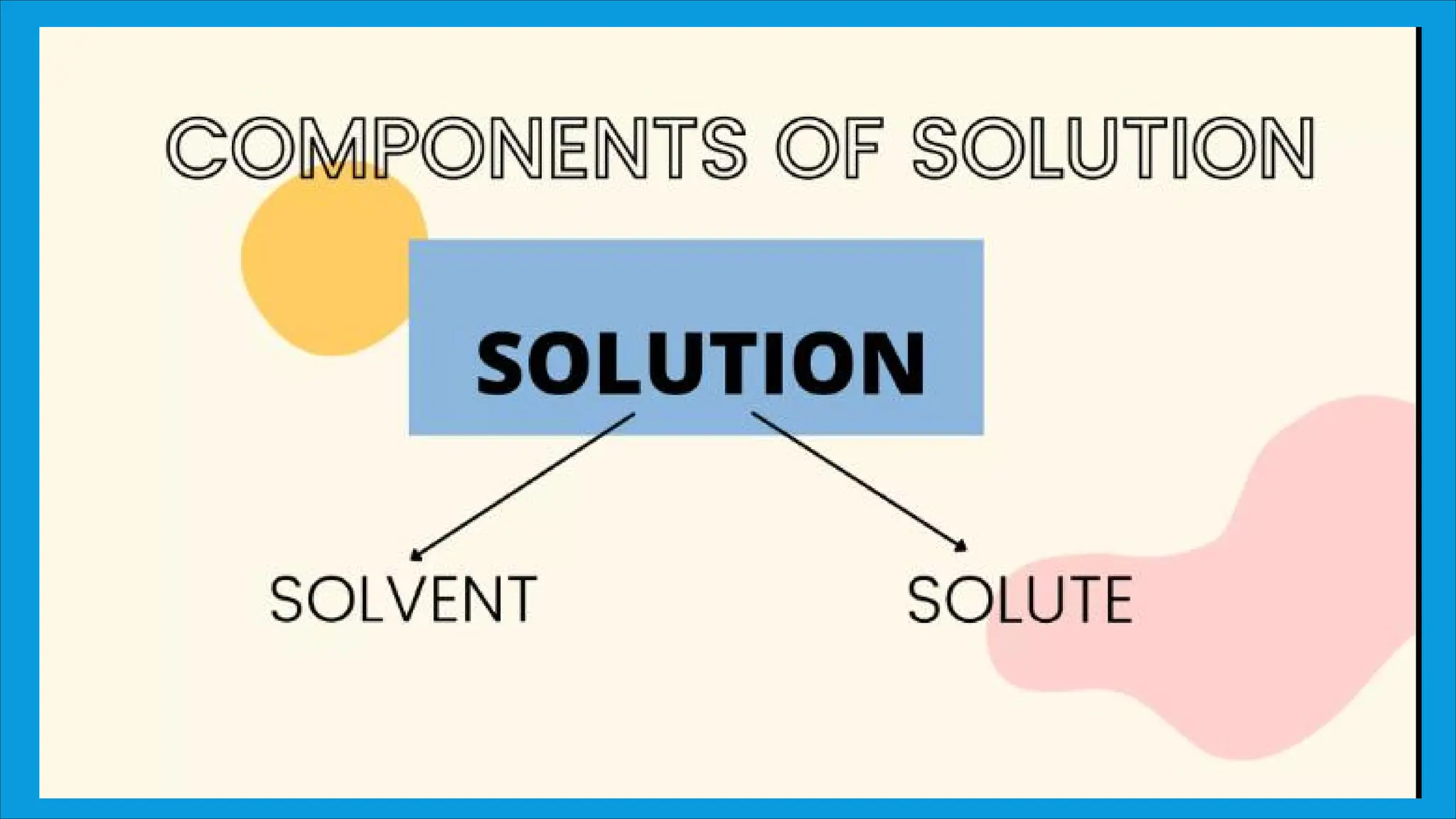
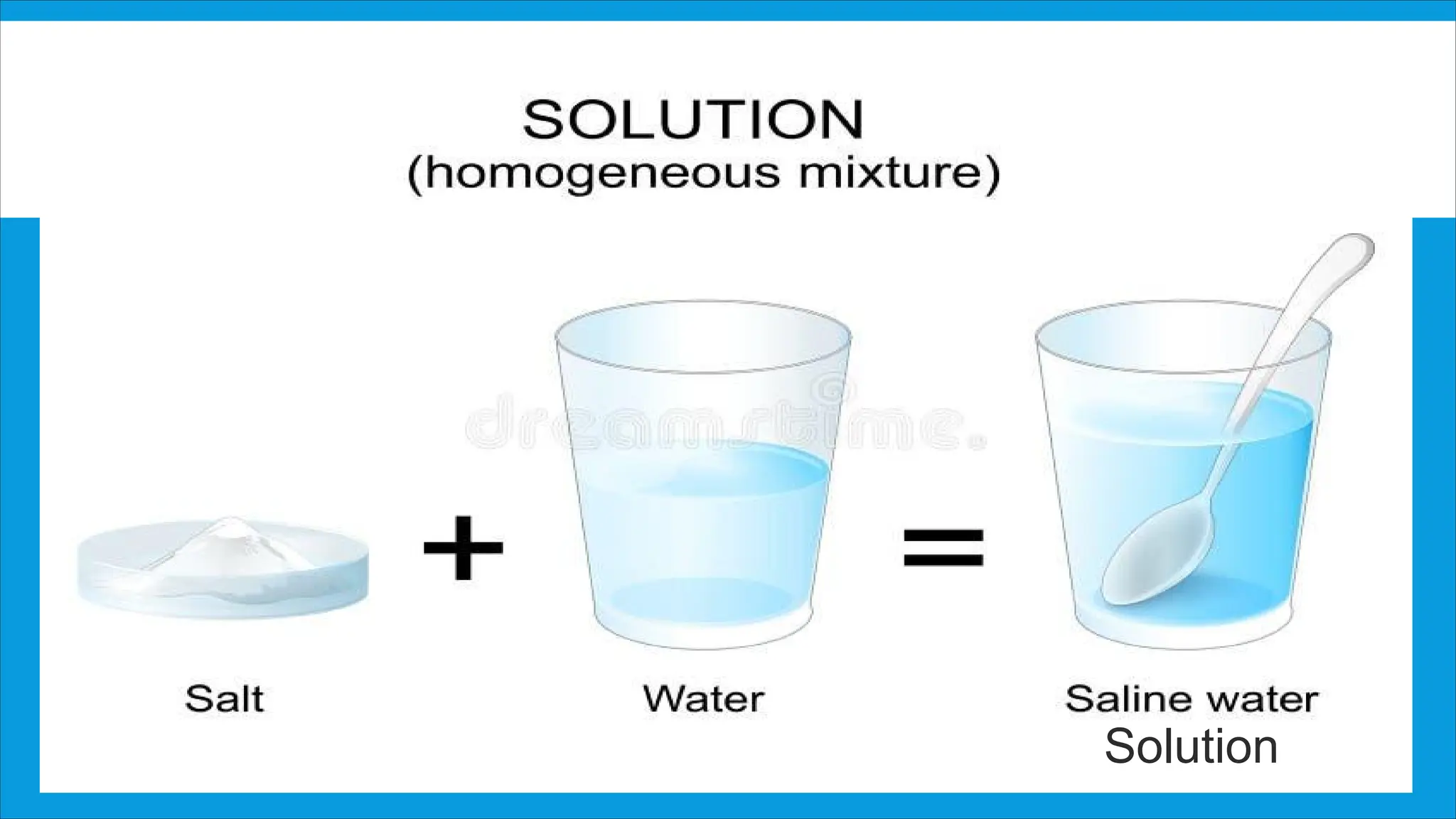
An unsaturated solution can dissolve more solute, a saturated solution holds the maximum amount of dissolved solute without any remaining undissolved, and a supersaturated solution contains more solute than it normally could, making it unstable and prone to crystallization.