Embed presentation
Download to read offline


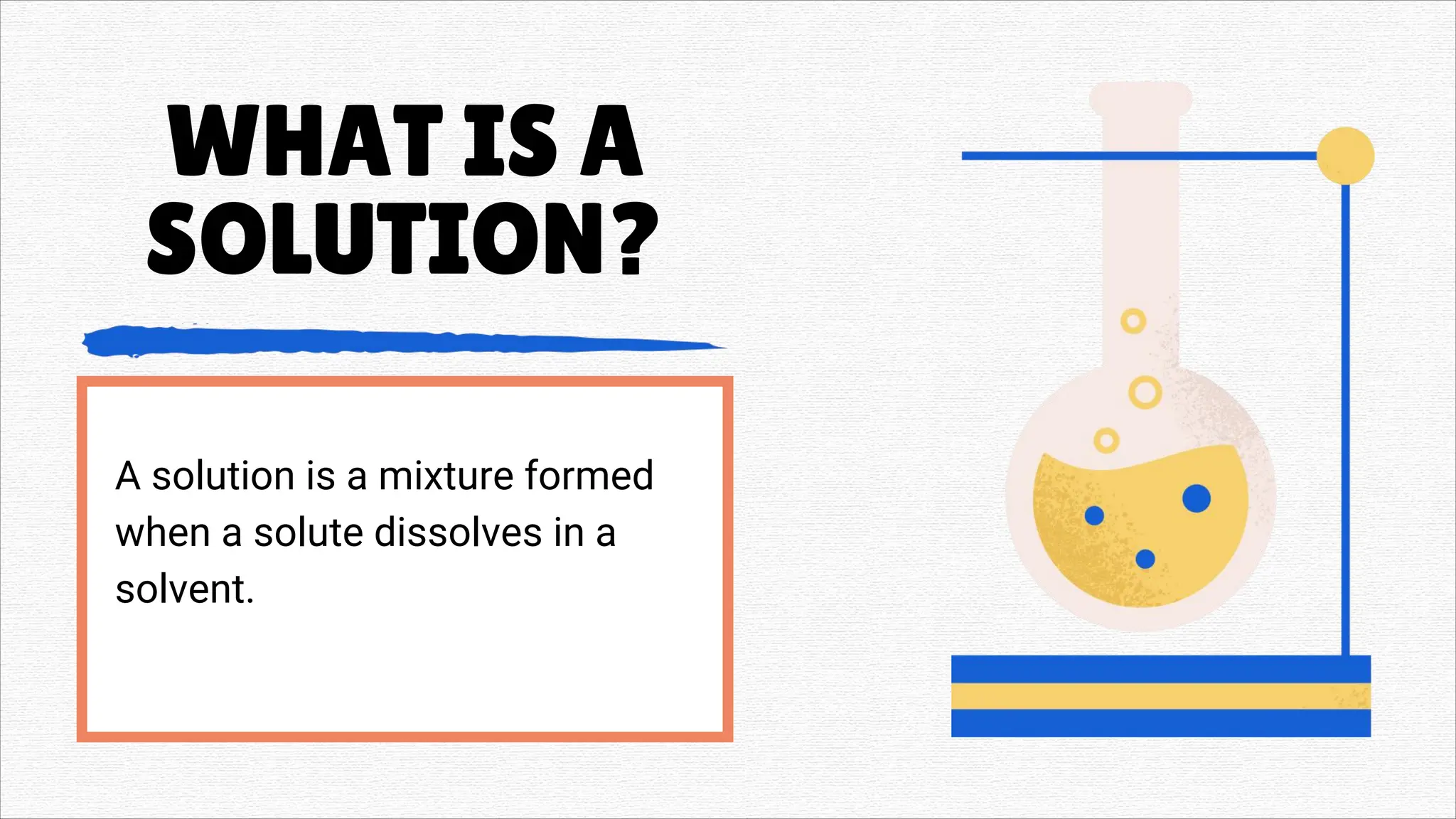
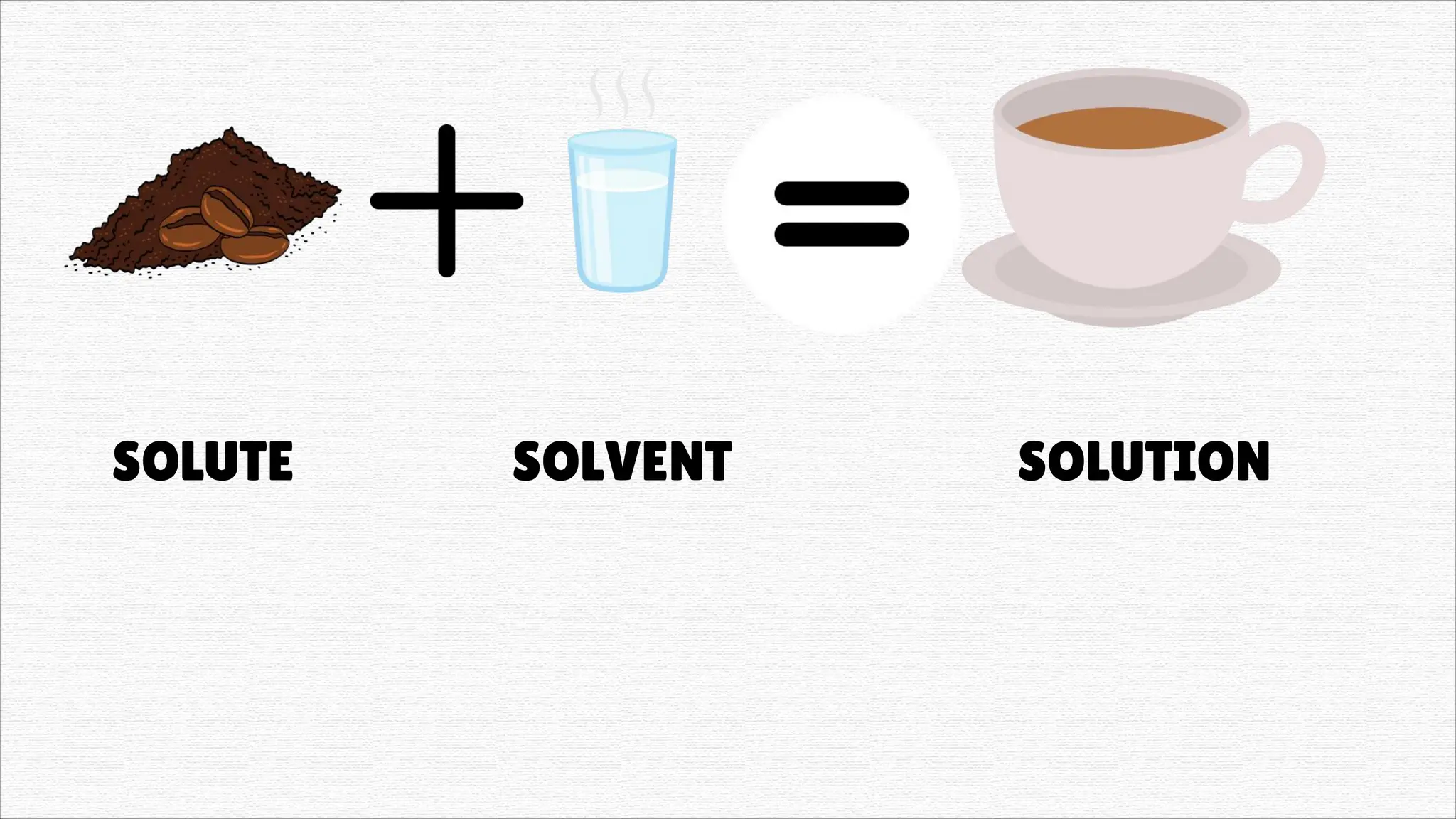
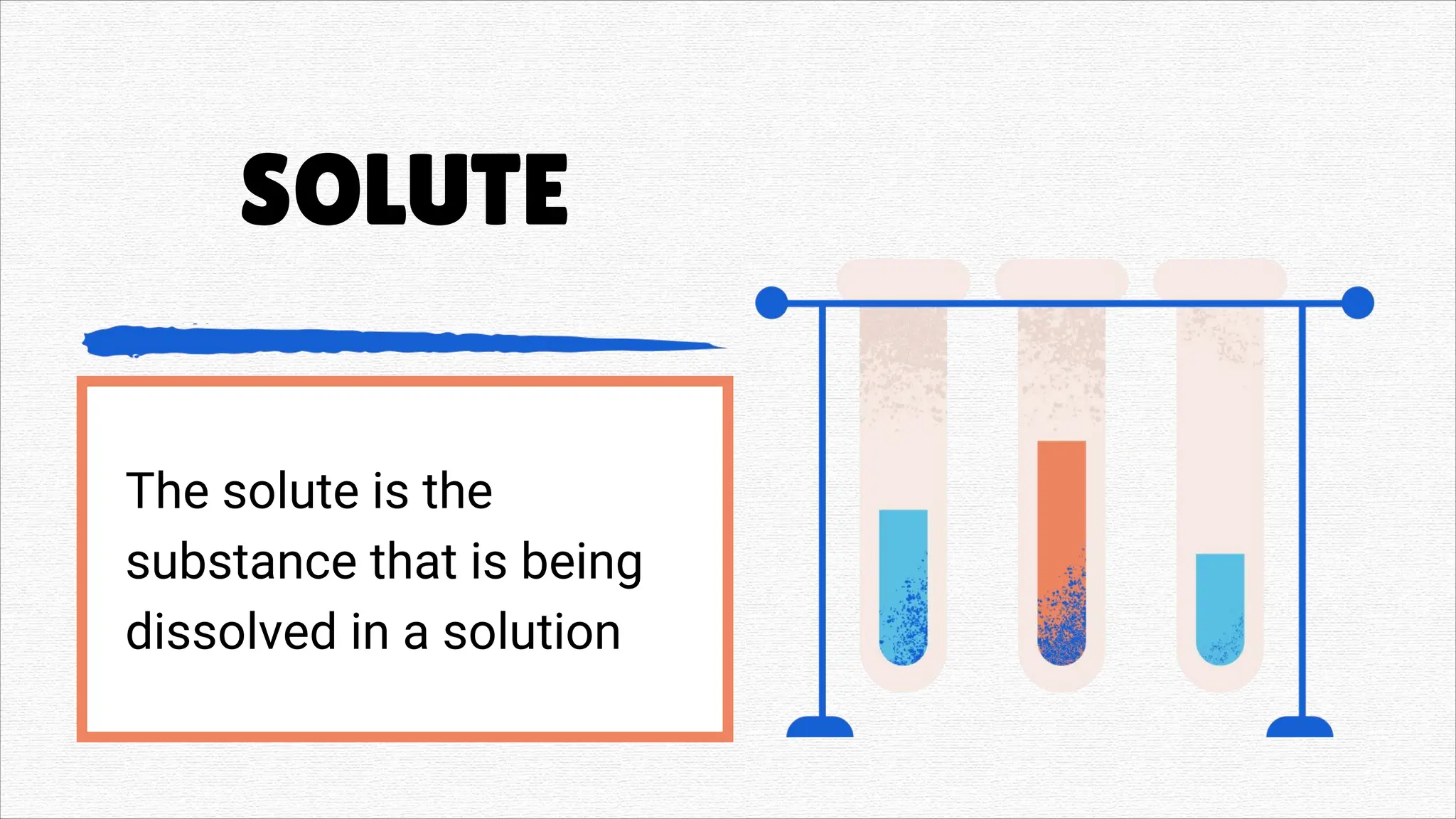

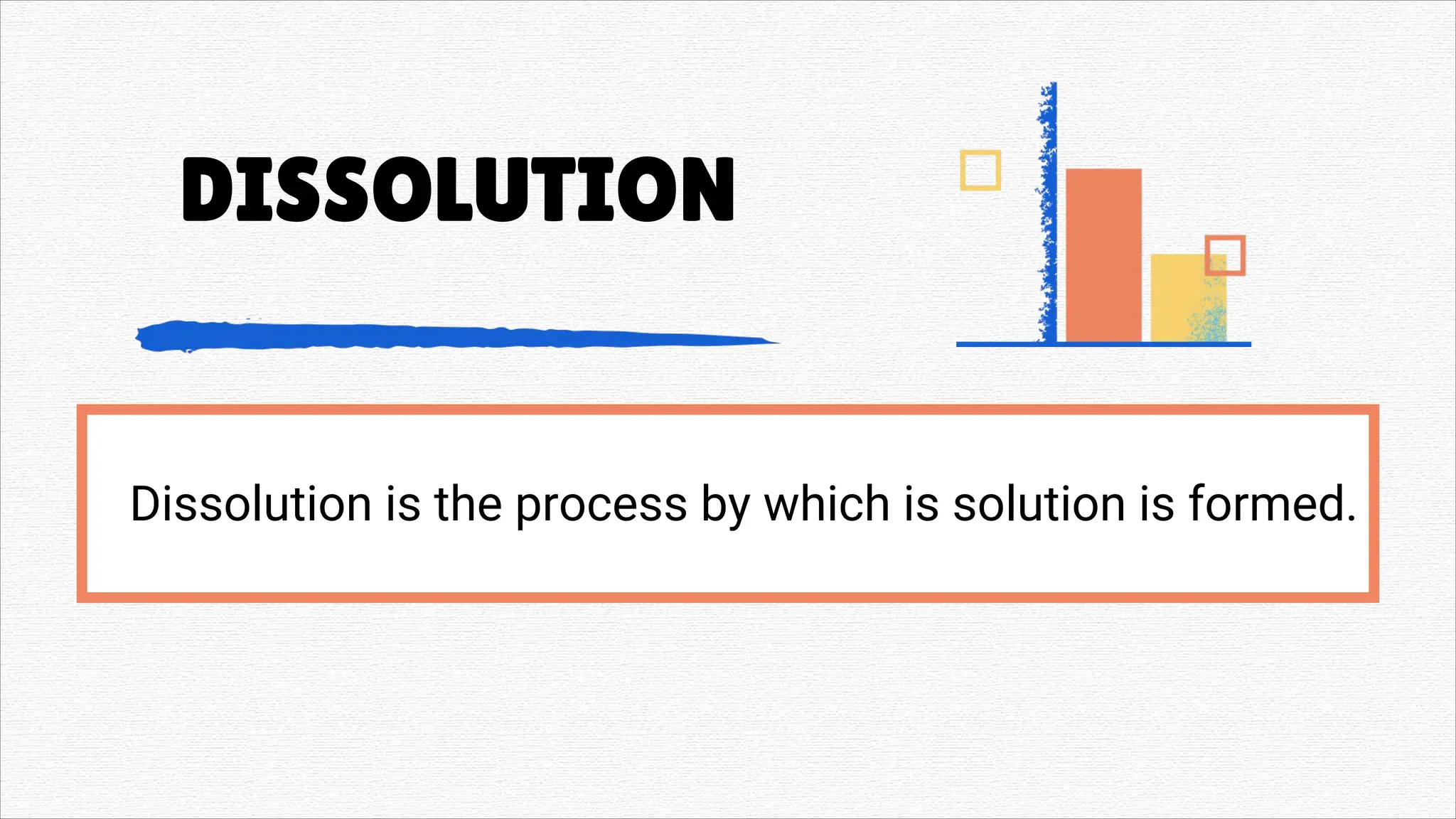







Solutions" refers to two main concepts: A solution in chemistry is a homogeneous mixture of two or more substances, like salt dissolved in water, where one substance (the solute) is dissolved uniformly in another (the solvent). A solution in a general sense is a means of solving a problem or dealing with a difficult situation, providing an answer or explanation.













