SBR is a synthetic rubber produced from styrene and butadiene monomers. It has good abrasion resistance and crack resistance and is widely used in automobile and truck tires. SBR can be produced via emulsion or solution polymerization processes.
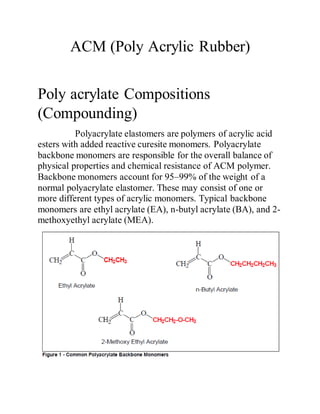
ACM is a synthetic rubber produced from acrylate monomers like ethyl acrylate, butyl acrylate, and methoxyethyl acrylate. It has good oil and temperature resistance properties. ACM is commonly used in automotive transmissions, hoses, seals, and mounts. It is produced via an emulsion polymerization process involving the addition of monomers and a chain transfer agent into a reactor over time.