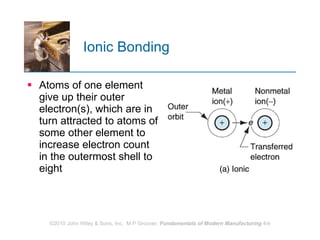
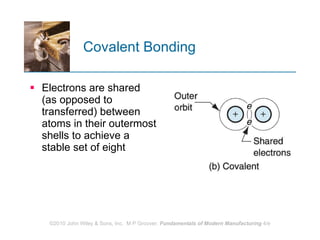
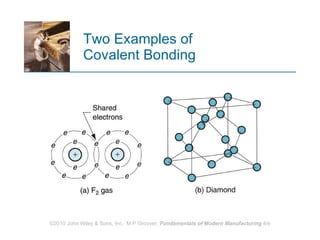
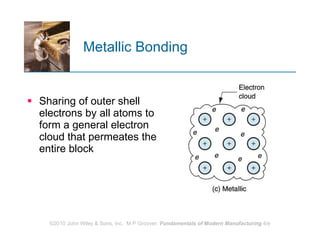
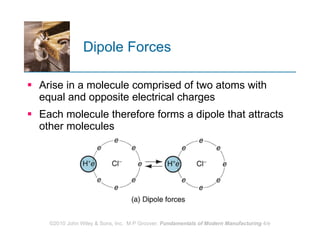
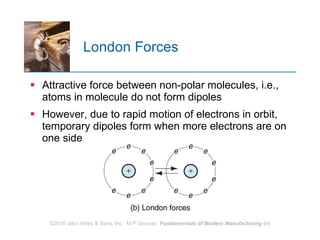
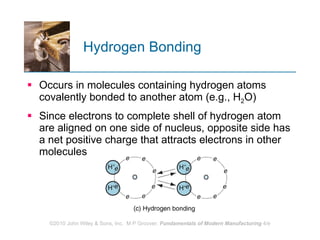
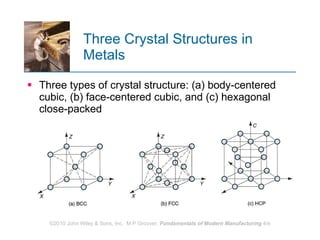
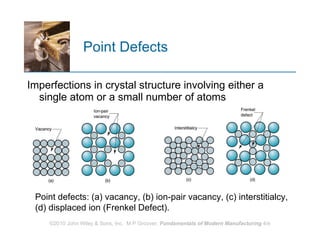
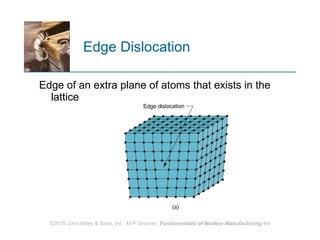
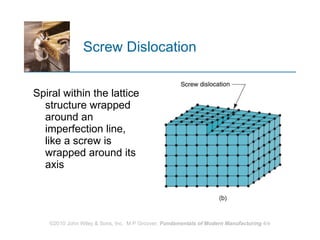
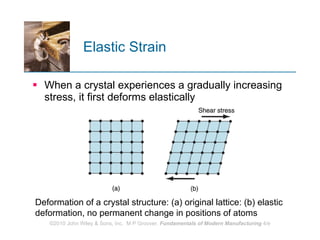
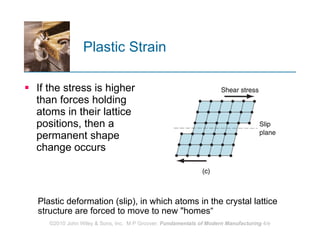
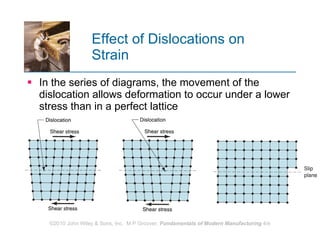
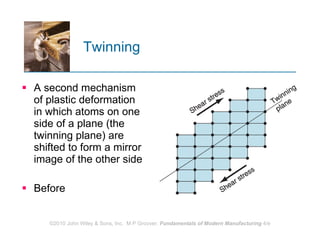
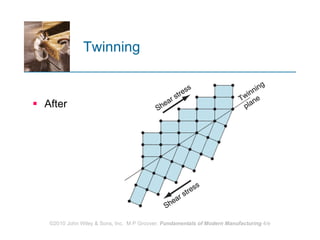
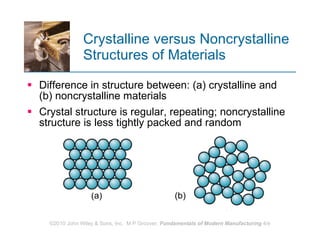
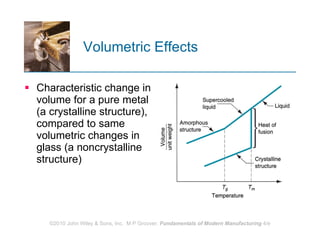
The document discusses the atomic structure and properties of materials. It describes how atoms are arranged in crystalline and noncrystalline structures and how different types of bonds like ionic, covalent and metallic bonds form between atoms. It explains defects in crystalline structures and how materials deform under stress. Metals typically have crystalline structures with metallic bonding while ceramics and polymers can have crystalline or noncrystalline structures with ionic/covalent bonding. The structures influence key material properties.