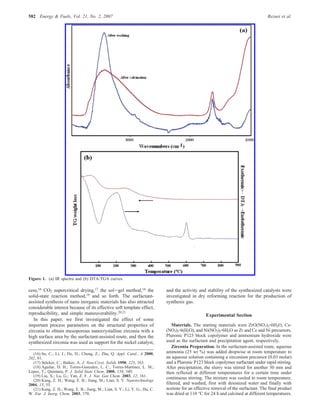
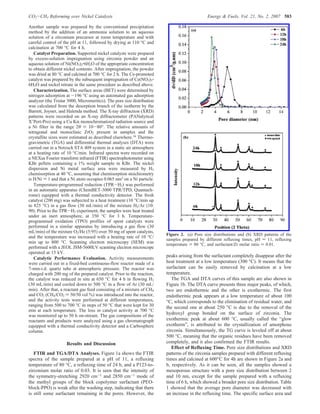
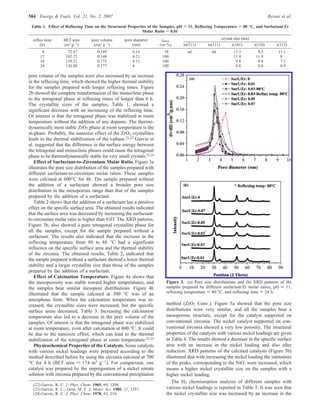
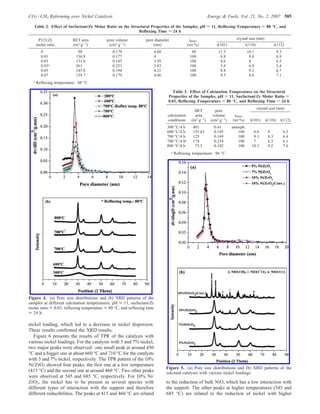
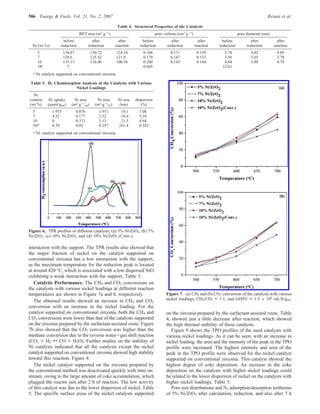
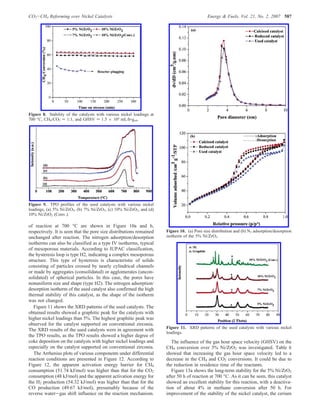
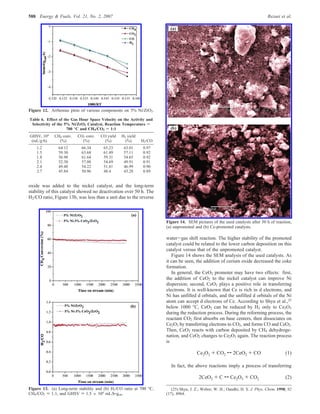
The document summarizes the preparation and characterization of mesoporous nanocrystalline zirconia with high surface area using a surfactant-assisted route. Nickel catalysts supported on the synthesized zirconia were then evaluated for dry reforming of methane to produce synthesis gas. The 5% Ni/ZrO2 catalyst exhibited stable activity for syngas production with only a 4% decrease in methane conversion after 50 hours of reaction. The addition of cerium oxide to the nickel catalyst further improved stability and decreased coke deposition.