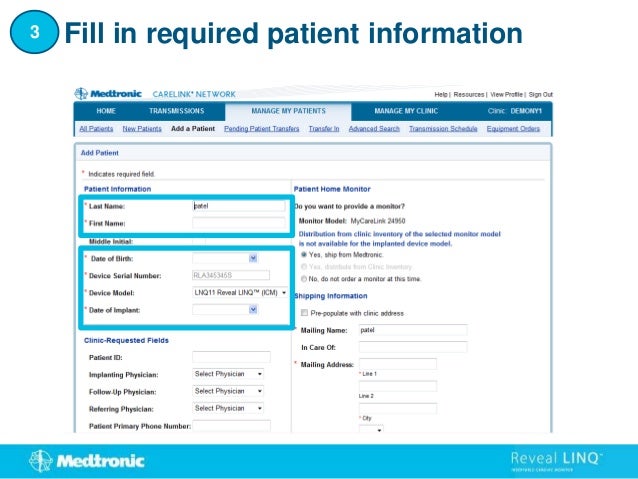
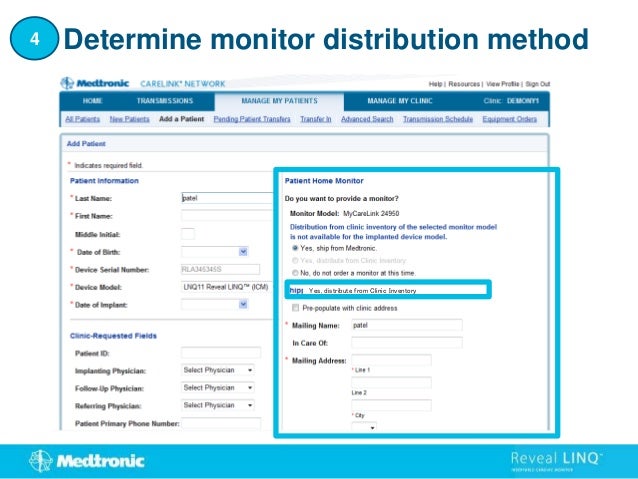
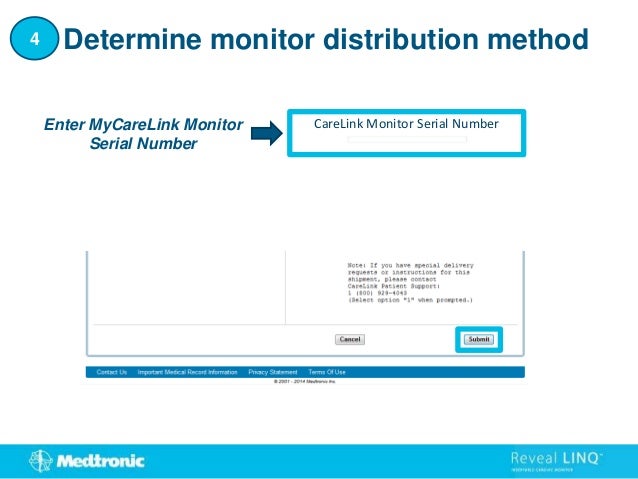
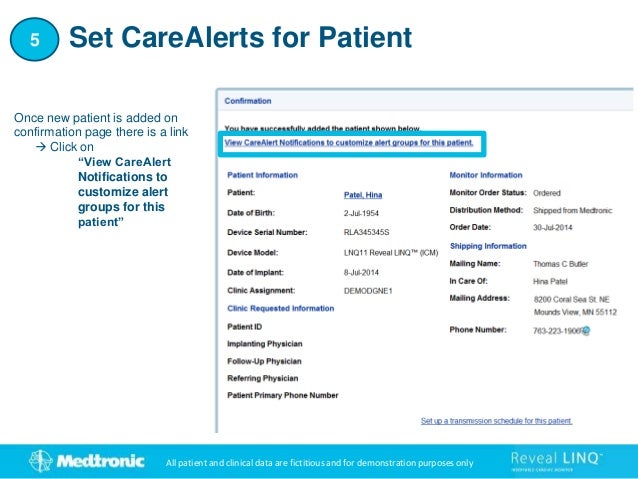
1. This document provides step-by-step instructions for connecting patients with a Reveal LINQ implantable cardiac monitor to the CareLink Network.
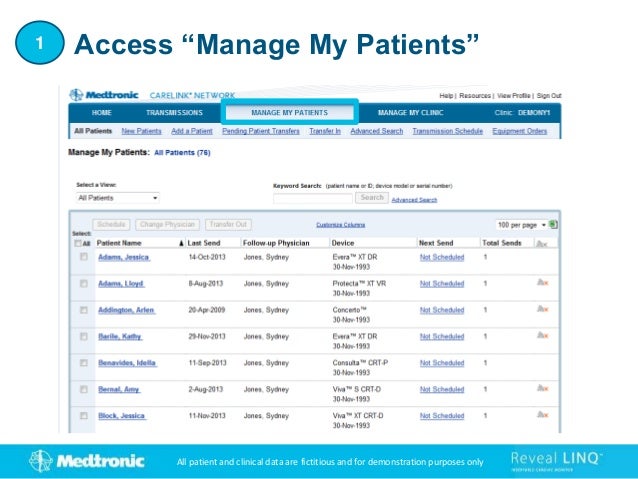
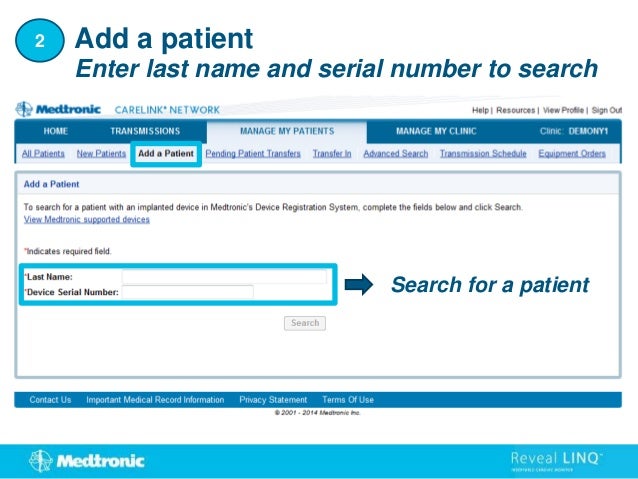
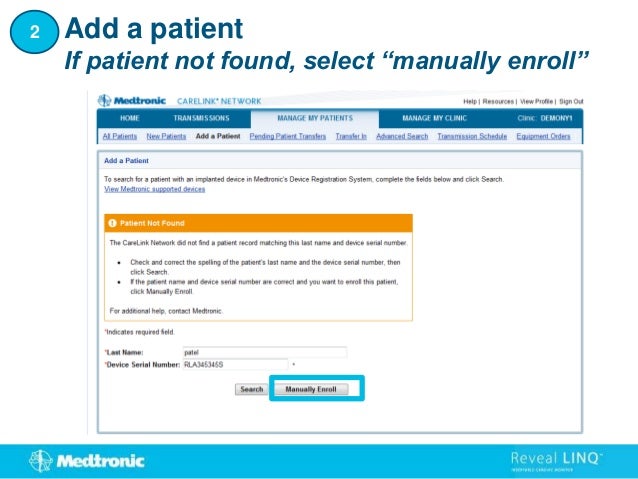
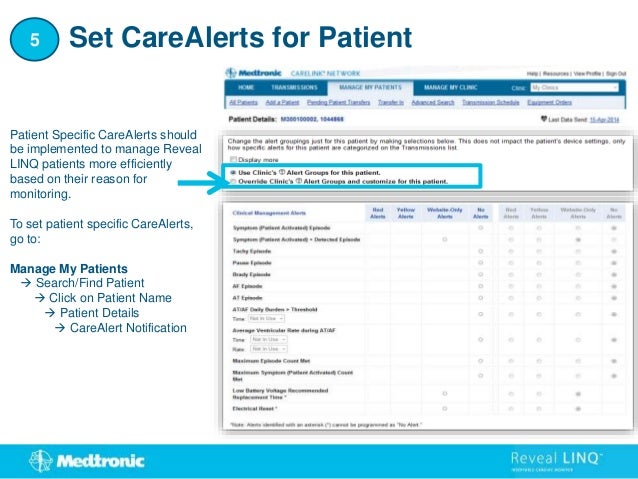
2. It outlines how to search for existing patients, manually enroll new patients, distribute monitors, and set customized alert groups for each patient.
3. The intended use, contraindications, warnings, and precautions are also described for the Reveal LINQ monitor, Patient Assistant device, and CareLink Network.