This document provides a multi-part assignment problem involving distillation column design and analysis. In part 1, the document summarizes the calculation of the number of theoretical plates and feed location for a binary distillation column separating benzene and toluene using the McCabe-Thiele method. The summary finds that the column requires 7 plates with the feed on plate 4. Part 2 involves using the Fenske equation to determine the minimum number of plates and minimum reflux ratio for a pentane-hexane separation. The summary states that the minimum number of plates is 4 and the minimum reflux ratio is 0.9024. Part 3 involves calculations to determine the allowable vapor velocity, column diameter, pressure drop per plate


![Assignment#2 Separation processes
Department of Chemical Engineering
Wah Engineering College.
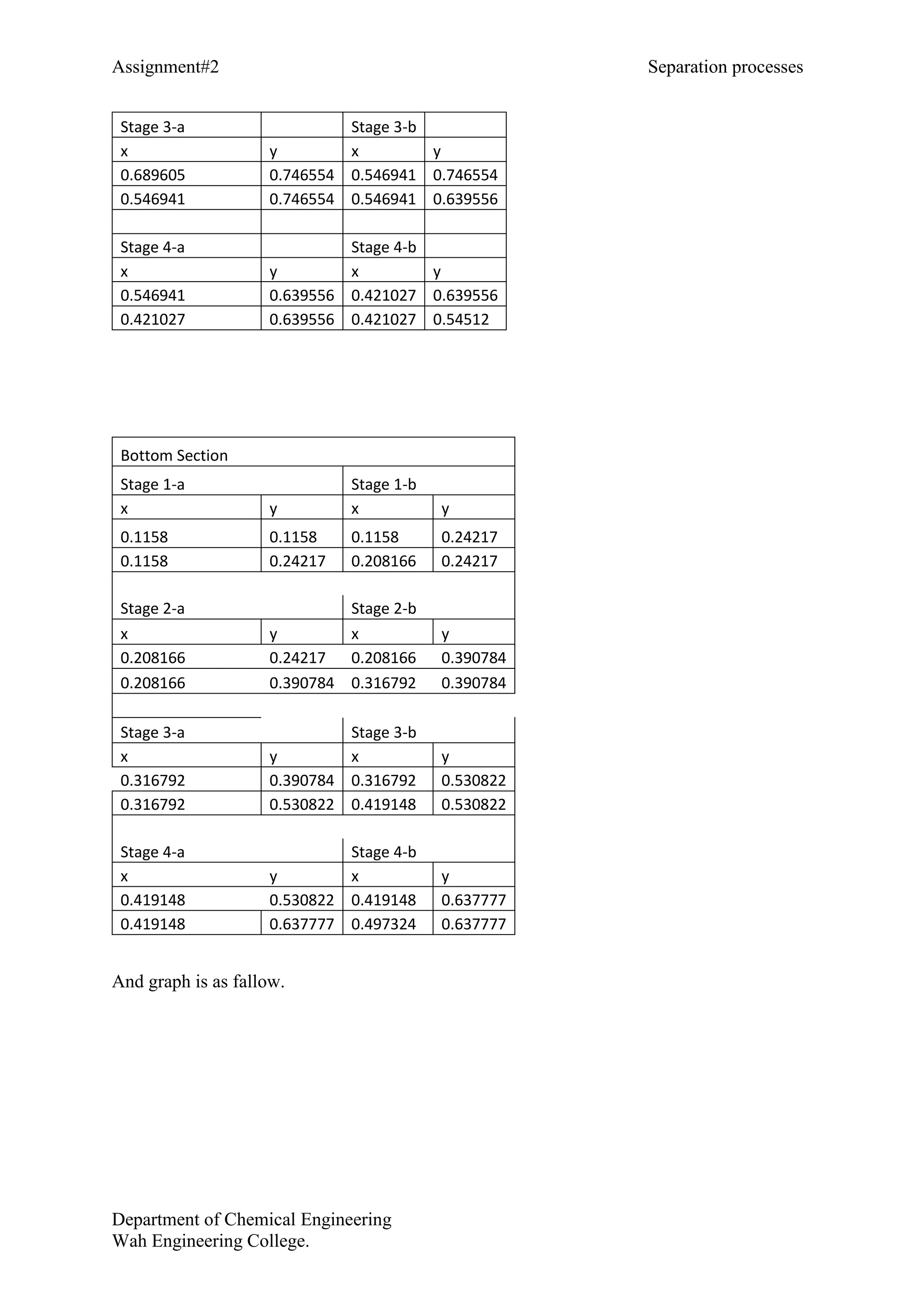
Number of theoretical plates = 7
Feed plate = 4
Question#2:
A distillation column receives a feed that is 40 mole % n-pentane and 60 mole % n-hexane.
Feed is saturated liquid with a flow rate of 2,500 lbmol/hr. The column is at 1 atm. A
distillate of 90 mole % n-pentane is desired. A total condenser is used. Reflux is a saturated
liquid. A bottom from the reboiler is 98 mole % n-hexane. Determine the minimum number of
equilibrium trays and the minimum reflux ratio.
Data: Vapor pressure, Psat, data: ln Psat = A - B/ (T + C), where Psat is in kPa and T is in K.
Compound A B C
n-pentane (1) 13.9778 2554.6 - 36.2529
n-hexane (2) 14.0568 2825.42 - 42.7089
Heat of evaporation for n-pentane, = 11,369 Btu/lbmol, CpL,C5 = 39.7 Btu/lbmol×o
F
Heat of evaporation for n-hexane, = 13,572 Btu/lbmol, CpL,C6 = 51.7 Btu/lbmol×o
F
𝛼 𝐷 = 3.1102
𝛼 𝐵 = 2.7955
Solution:
As total condenser is used so minimum number of trays can be find by Fenske equation
1
log[ * ]
1
log( )
D B
D B
m
ave
X X
X X
N
a
0
0.2
0.4
0.6
0.8
1
0 0.2 0.4 0.6 0.8 1](https://image.slidesharecdn.com/assignment2-160405212353/75/pressure-drop-calculation-in-sieve-plate-distillation-column-4-2048.jpg)
![Assignment#2 Separation processes
Department of Chemical Engineering
Wah Engineering College.
𝛼 𝐷 = 3.1102
𝛼 𝐵 = 2.7955
1
2
1
2
( * )
(3.1102*2.7955)
2.9486
ave D B
ave
ave
a a a
a
a
1 0.9 1 0.1log[ * ] log( * )
1 1 .9 0.1
log( ) log(2.9486)
log(81)
log(2.9486)
4.1
D B
D B
m
ave
m
m
X X
X X
N
a
N
N
Minimum reflux ratio
*(1 )1
[ ( )]
1 (1 )
1 0.9 2.9486*(1 0.9)
[ ( )]
2.9486 1 0.4 (1 0.4)
0.9024
ave DD
m
ave F F
m
m
a XX
R
a X X
R
R
Question#3:
A sieve-plate column operating at atmospheric pressure is to produce nearly pure methanol
from an aqueous feed containing 40 mole percent methanol. The distillate product rate is 5800
kg/hr. (a) for a reflux ratio of 3.5 and a plate spacing of 18in, calculate the allowable vapor
velocity and column diameter. (b) calculate the pressure drop per plate if each sieve tray is
1
8
-in. thick with
1
4
-in. holes on a
3
4
-in. triangular spacing and a weir height of 2 in. (c) what is
the froth height in the downcomer ?
Solution:
Given data
5800 /
3.5
40 mole % methanol.
D kg hr
R
Physical properties of Methanol:
M.w = 32, normal boiling point 650
C
Density of liquid Methanol =ρL= 750 kg/m3
(650
C), surface tension= 19 dyn/cm.](https://image.slidesharecdn.com/assignment2-160405212353/75/pressure-drop-calculation-in-sieve-plate-distillation-column-5-2048.jpg)


