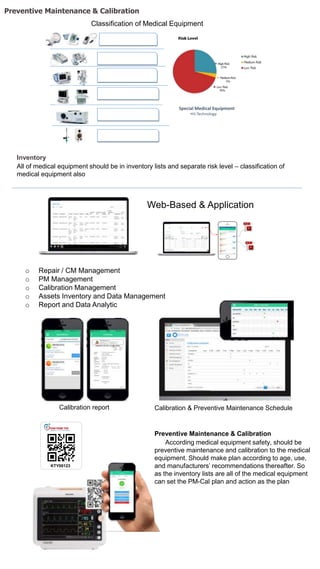
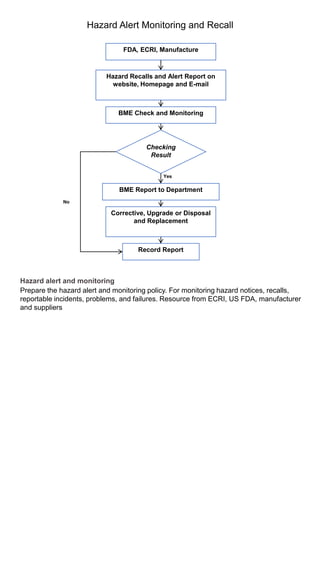
The document discusses medical equipment management standards and programs. It outlines requirements for hospitals to establish a medical equipment program that includes inventorying all equipment, inspecting and testing equipment on acquisition and according to manufacturers' recommendations, and including preventive maintenance. Staff must be qualified and trained. The program must also monitor for hazard notices, recalls, and incidents, and report any deaths or injuries resulting from equipment. Medical equipment is classified into various risk categories.