The document discusses several key concepts in physics:
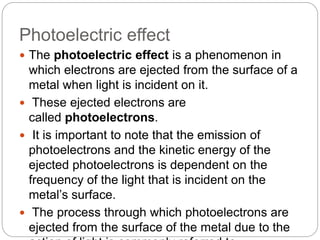
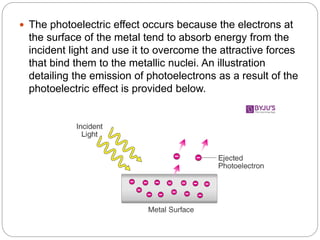
1) It describes the photoelectric effect where electrons are ejected from metals when light is incident, with the kinetic energy of ejected electrons dependent on the frequency of light.
2) It explains that for the photoelectric effect to occur, photons must have sufficient energy to overcome the attractive forces binding electrons. This minimum energy is called the threshold energy.
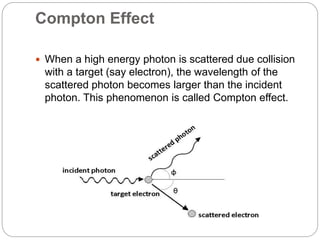
3) It briefly introduces the Compton effect where the wavelength of scattered photons increases due to collisions with electrons, and de Broglie waves which associate a wavelength to moving particles.


![de Broglie Waves
It is said that matter has a dual nature of wave particles .There is a
wave associated with every moving particle .This is known as de
Broglie matter wave. The wavelength of this wave is called de
Broglie wavelength.
We know that , if the frequency of a photon is f and it has energy of E,
The E= hf , where h= Planck's constant
Or f =
𝐸
ℎ
From relativity it is found that,
E= pc, Where p= linear momentum and c = speed f light
Or, hf = pc
Or, p=
𝑓ℎ
𝑐
Or, p =
ℎ
𝜆
, [𝜆 =
𝑐
𝑓
]
So, 𝜆 =
ℎ
𝑝](https://image.slidesharecdn.com/modernphysics-240229045716-018829ce/85/Photoelectric-Effect-Compton-Effect-X-ray-7-320.jpg)