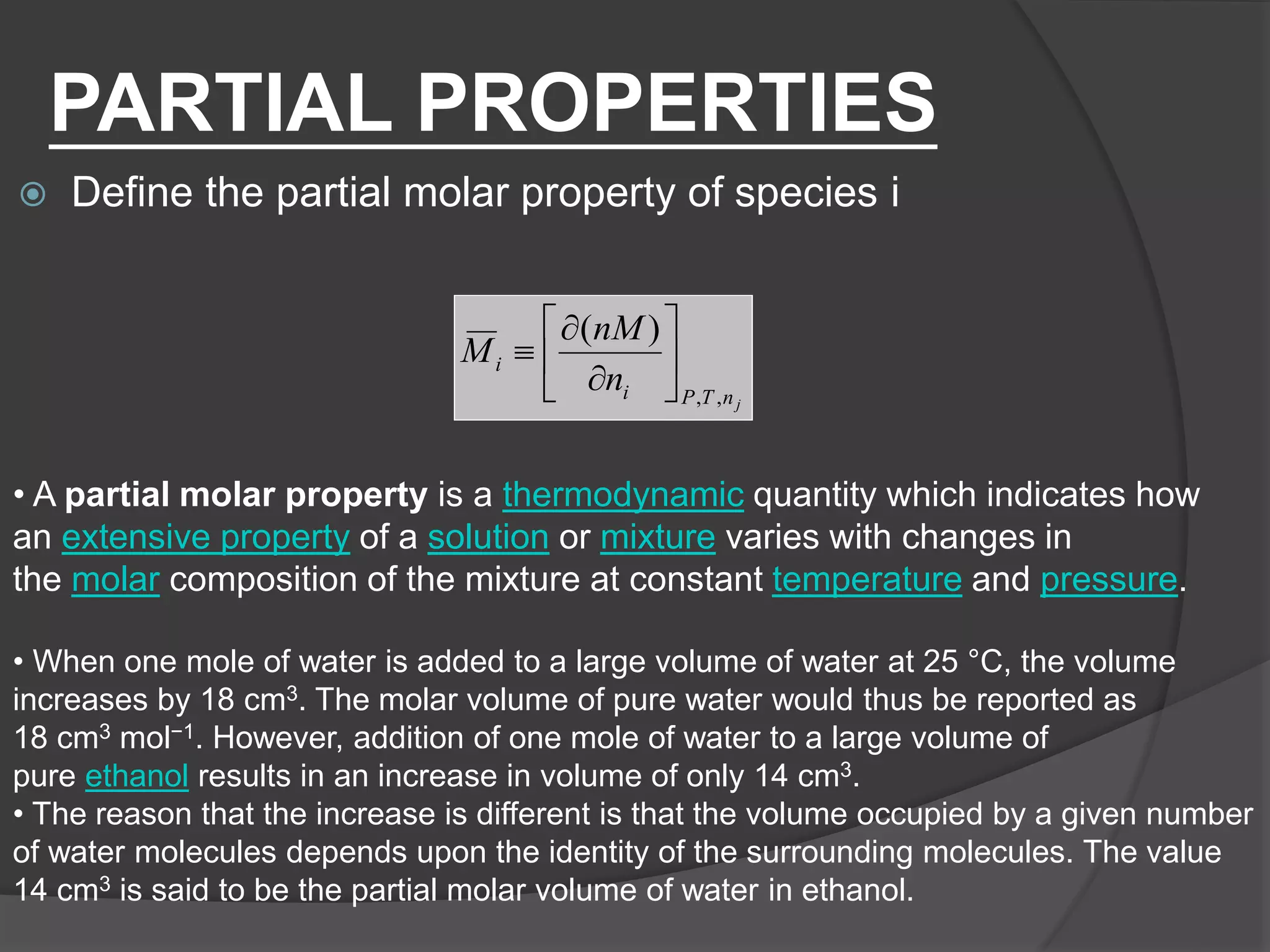
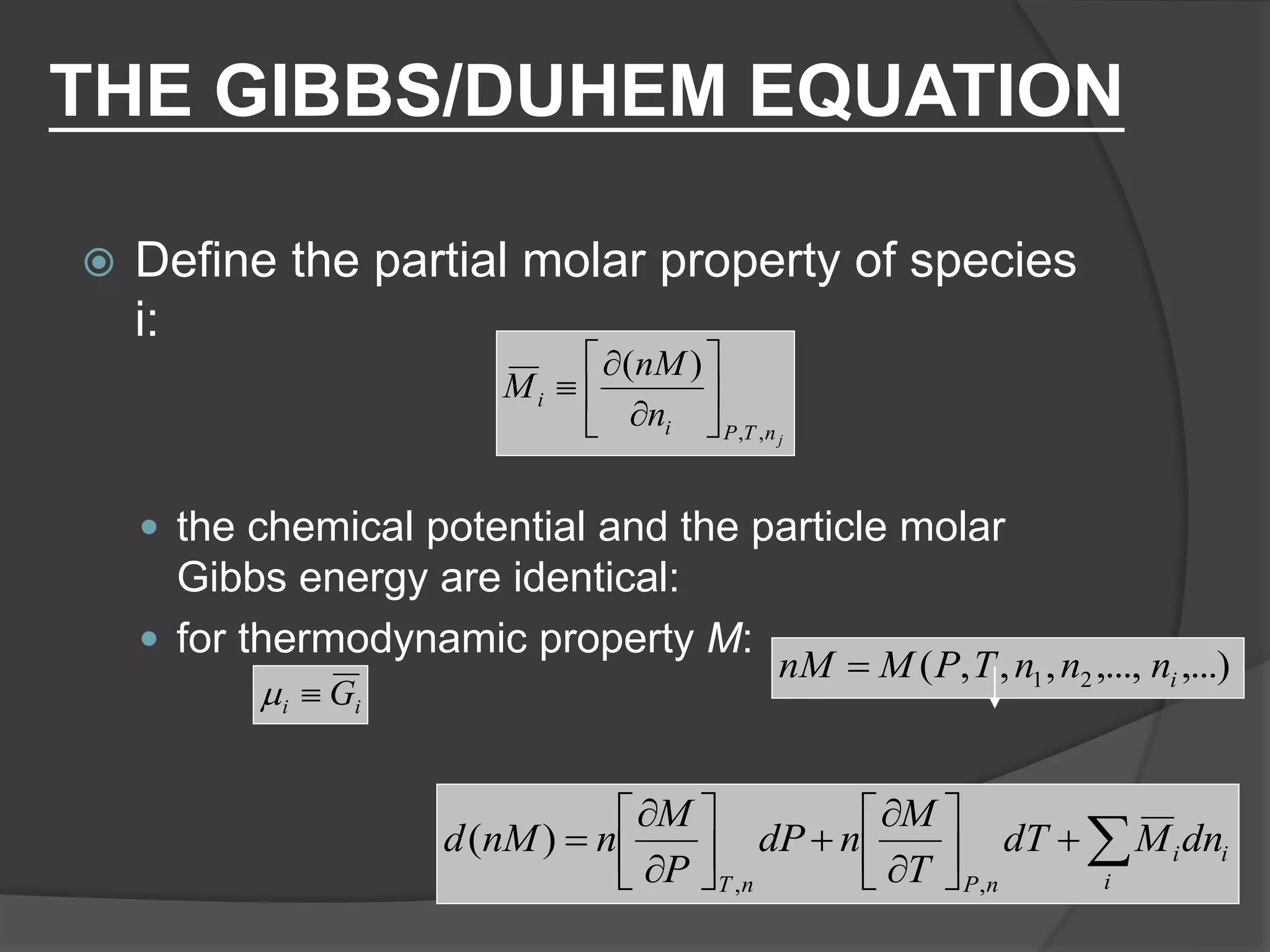
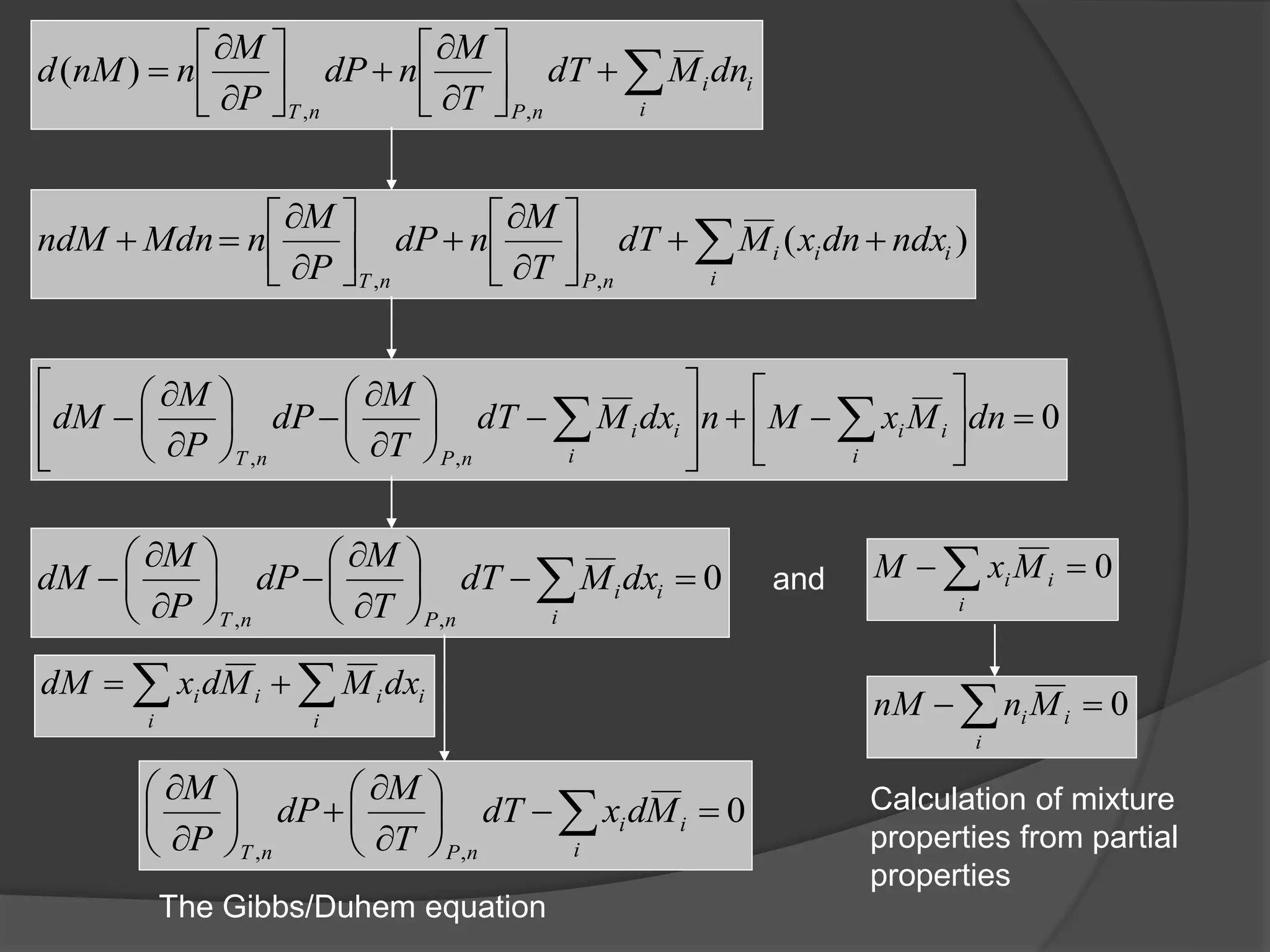
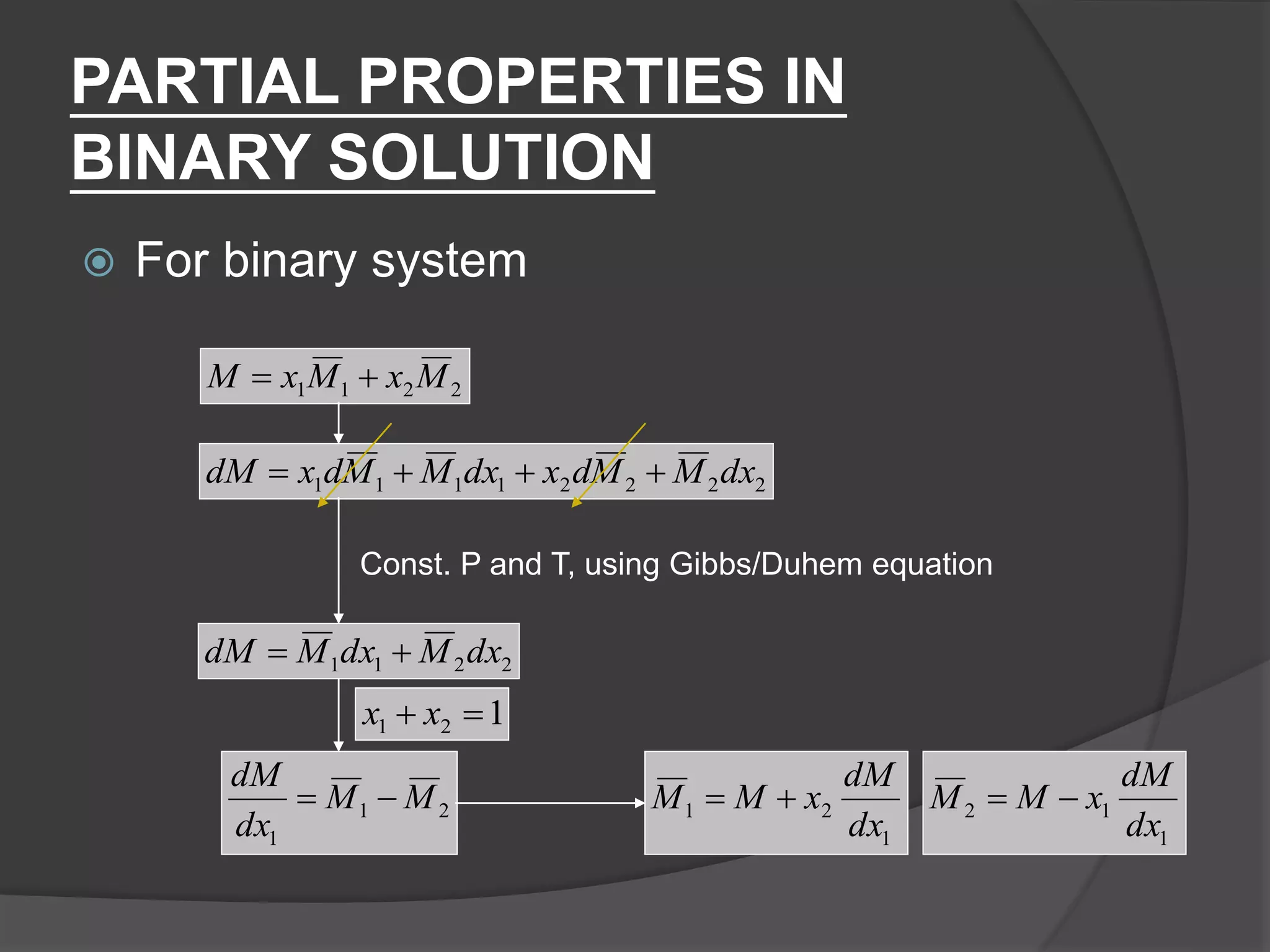
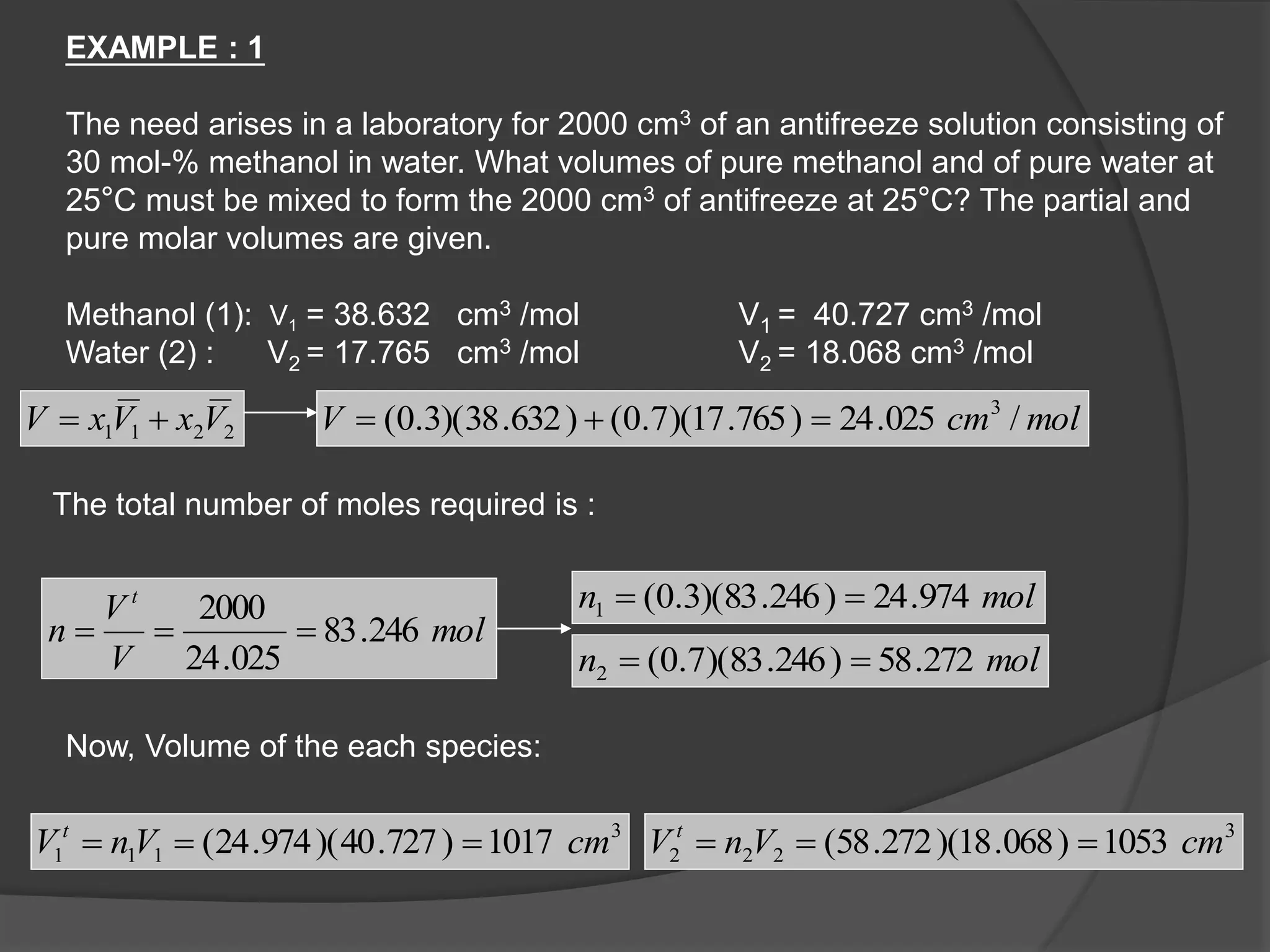
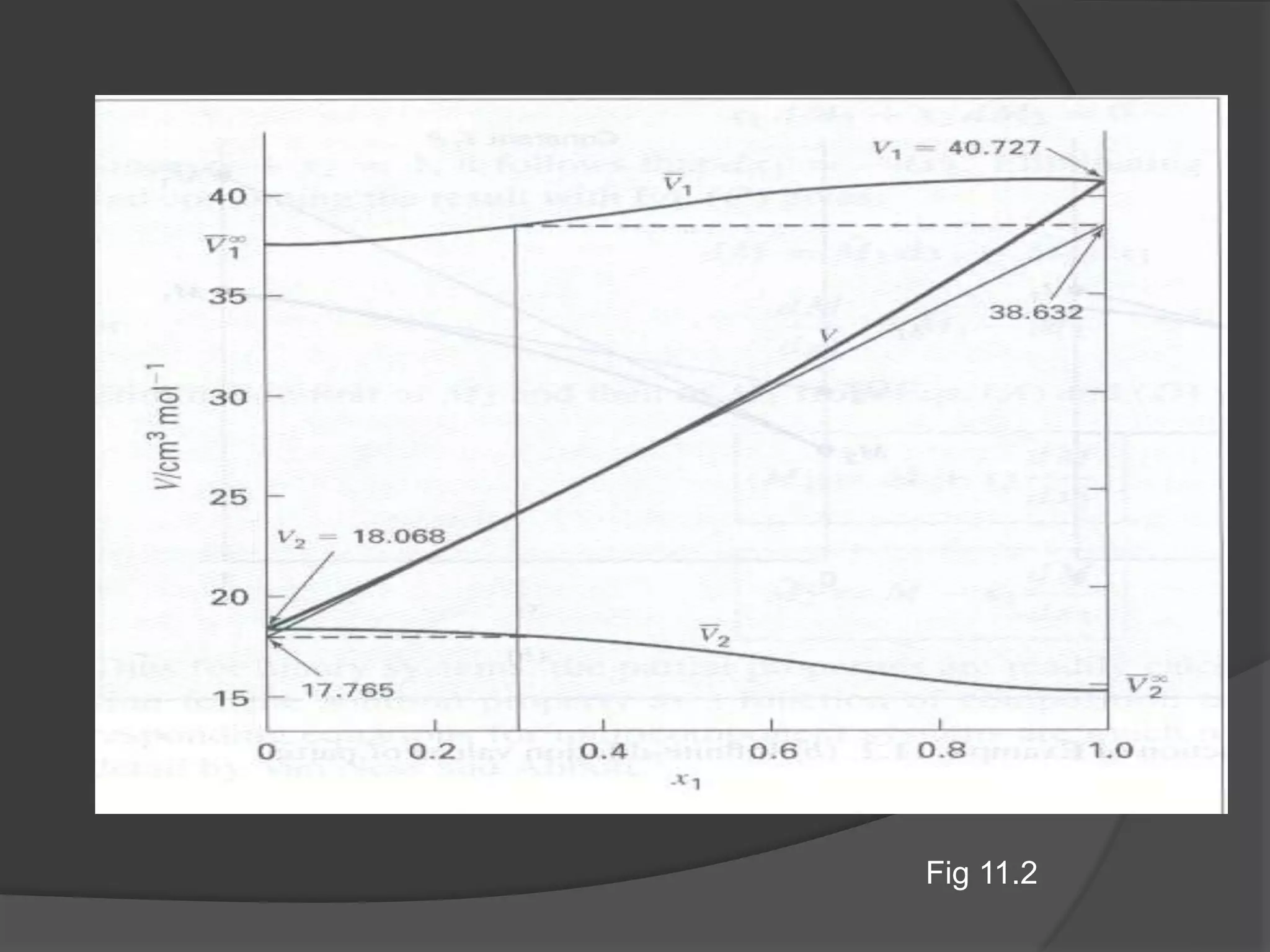
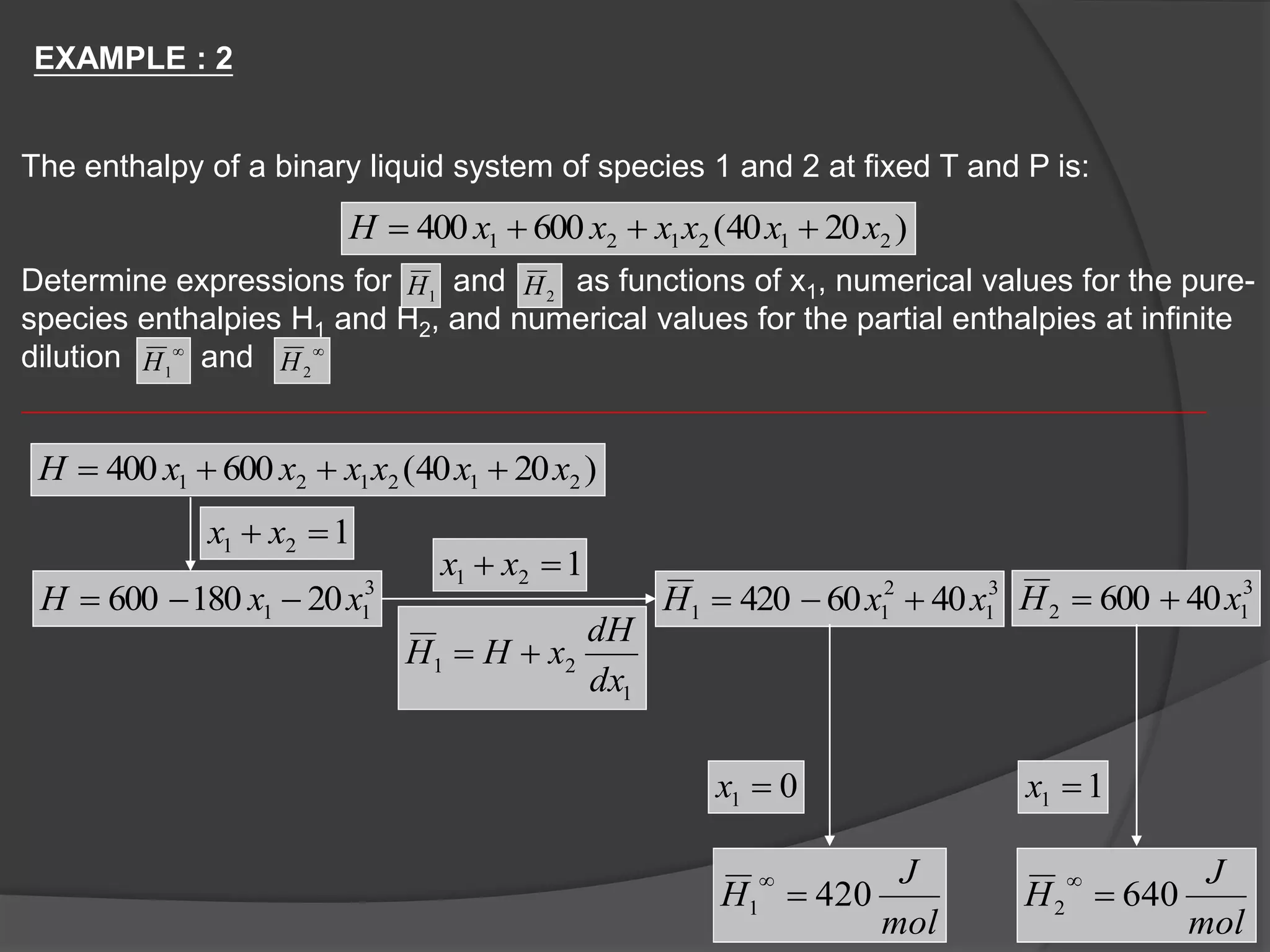
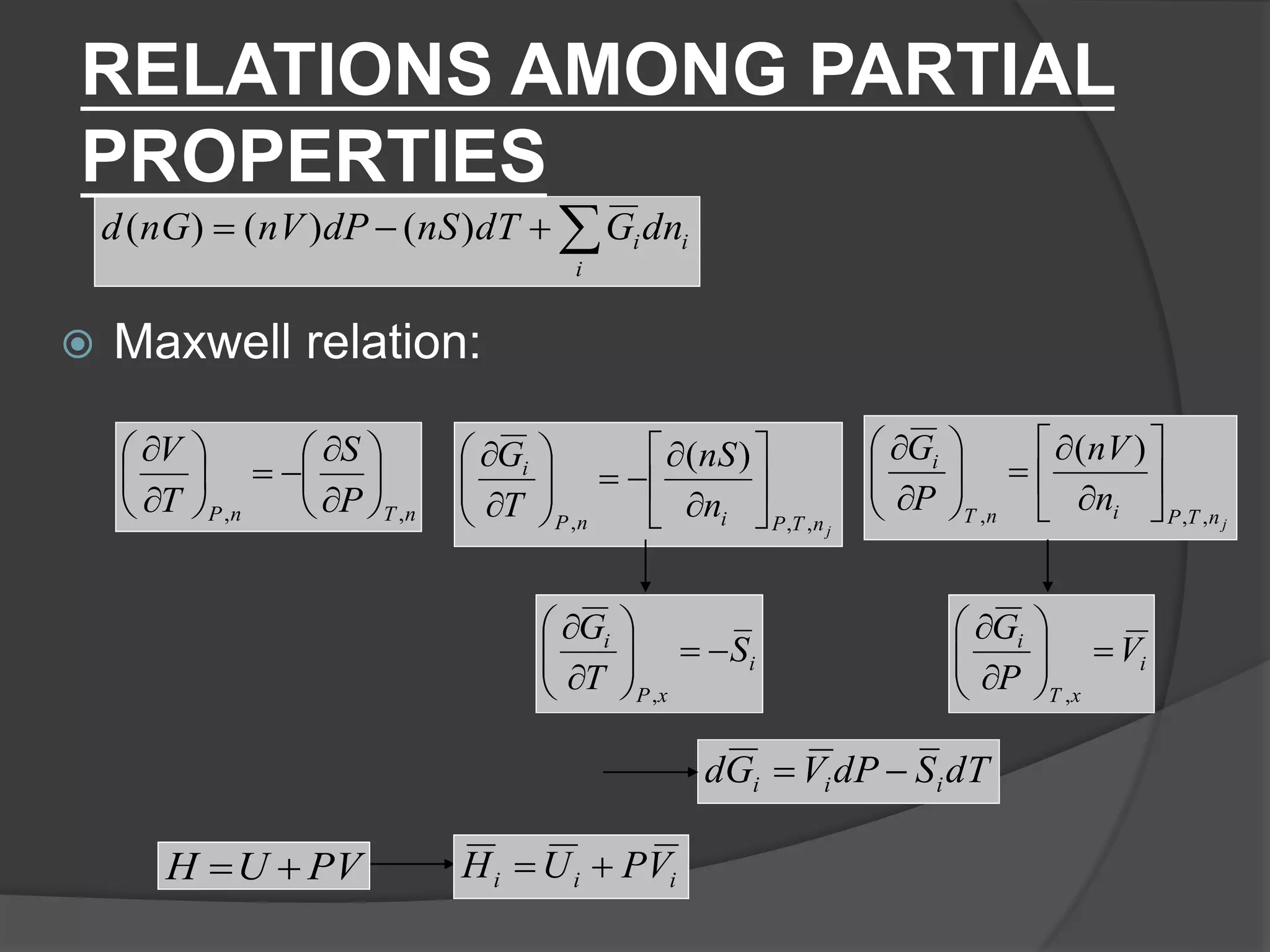
El documento describe las propiedades molares parciales de las especies en soluciones, destacando que estas propiedades termodinámicas reflejan cómo varía una propiedad extensa en función de la composición molar del sistema a temperatura y presión constantes. Se explican ejemplos con agua y etanol, así como el uso de la ecuación de Gibbs-Duhem para soluciones binarias y su influencia en puntos de ebullición y entalpías. También se presentan relaciones entre propiedades parciales mediante ecuaciones termodinámicas relevantes.