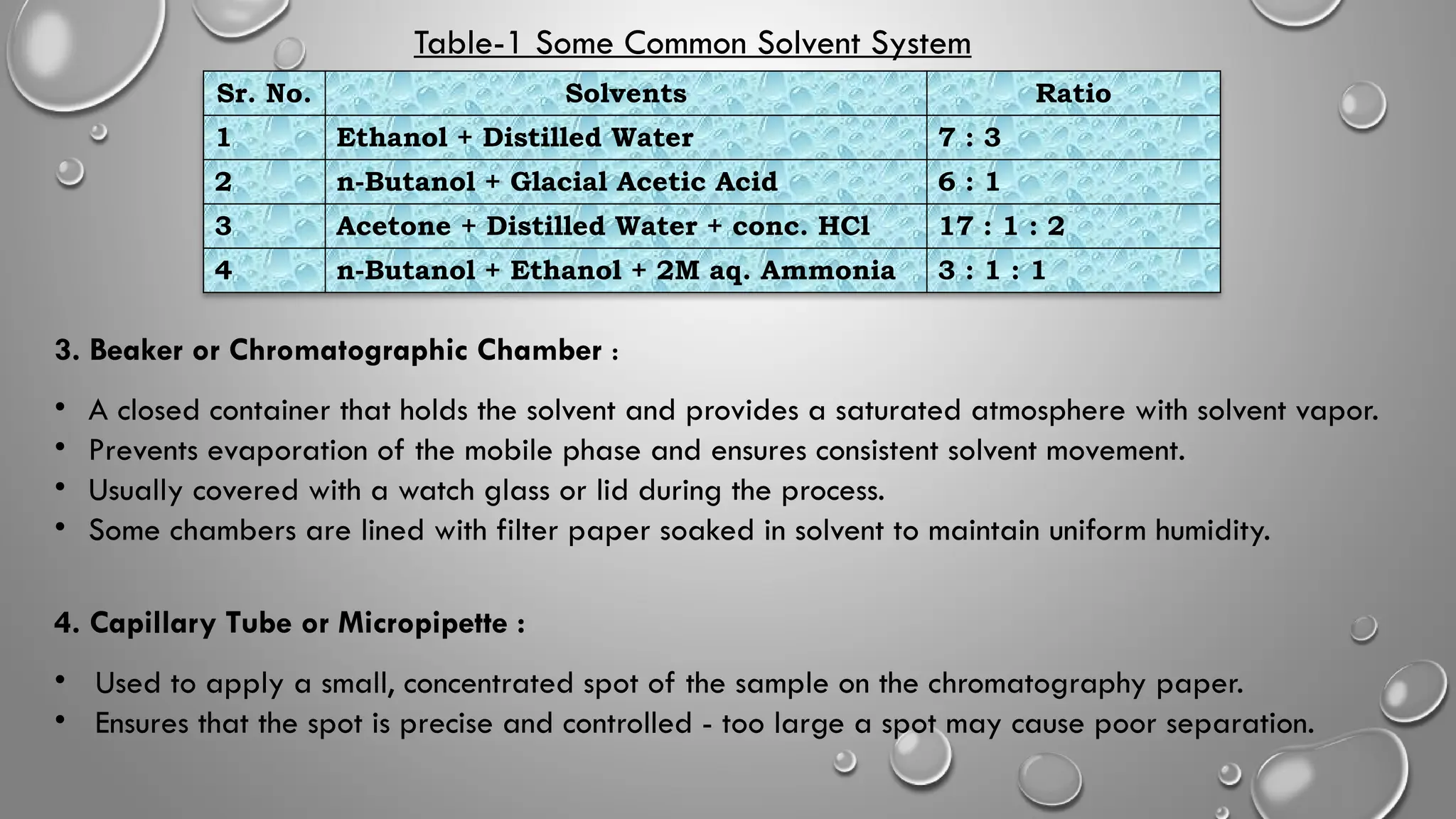
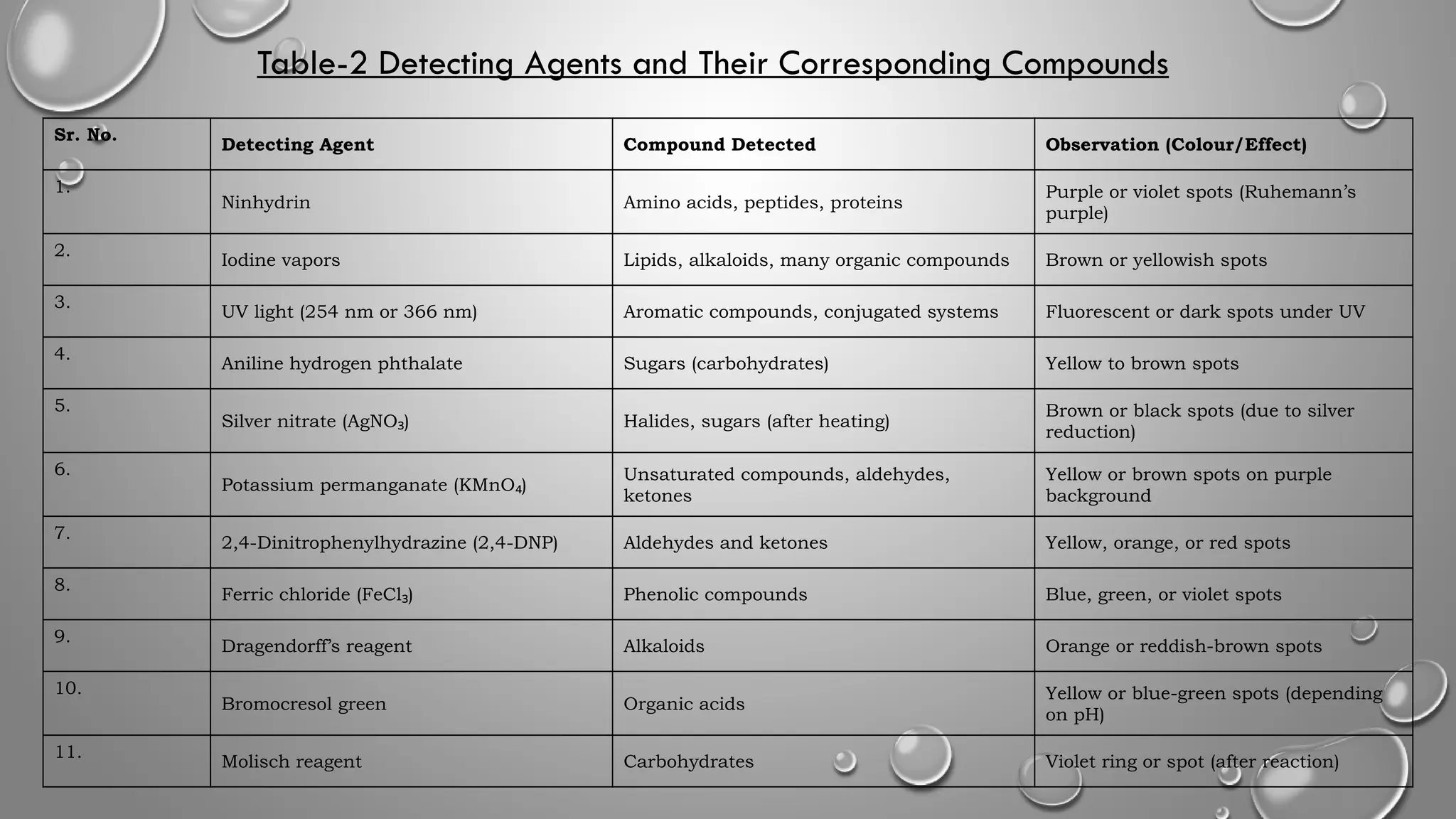
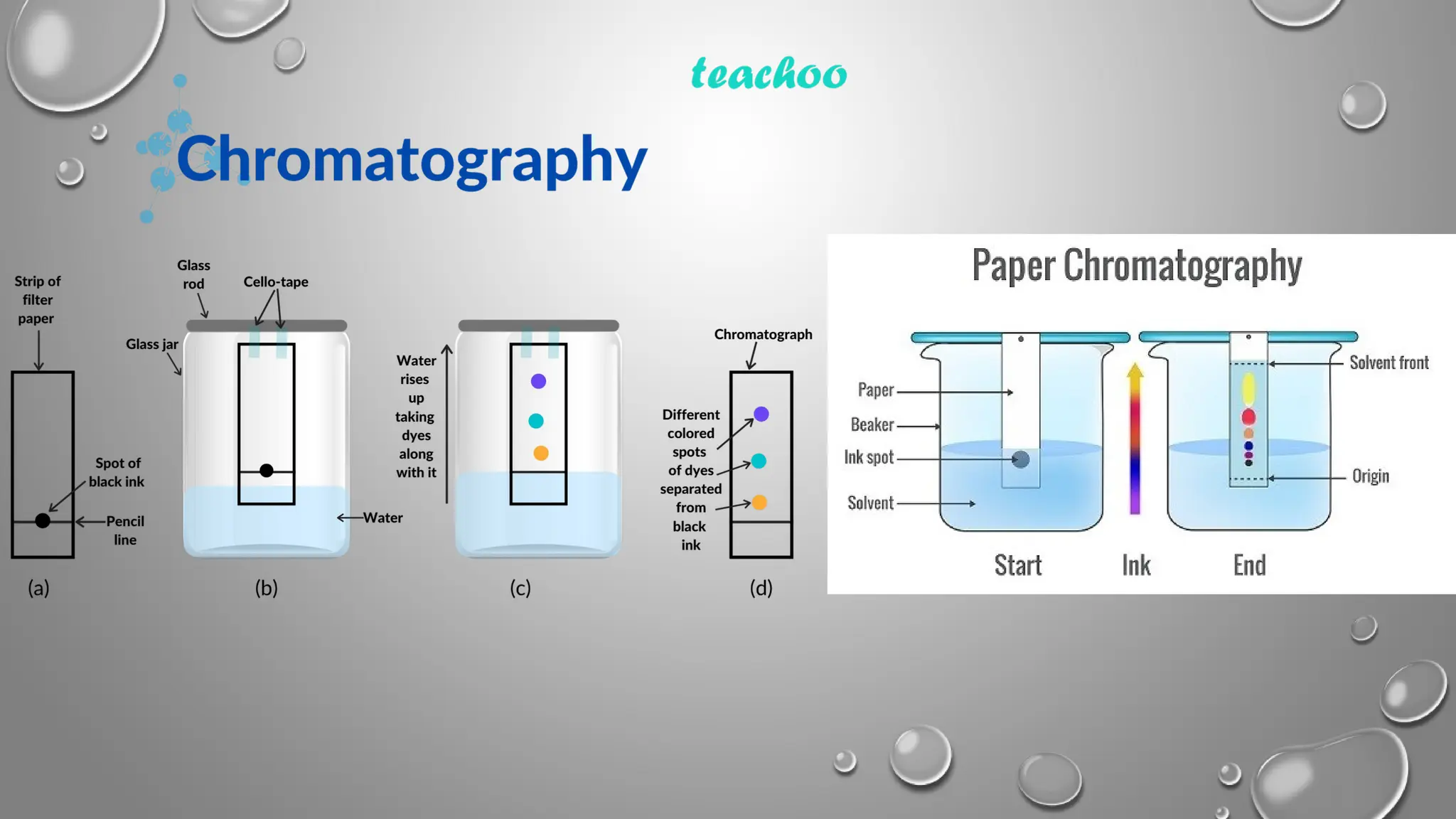
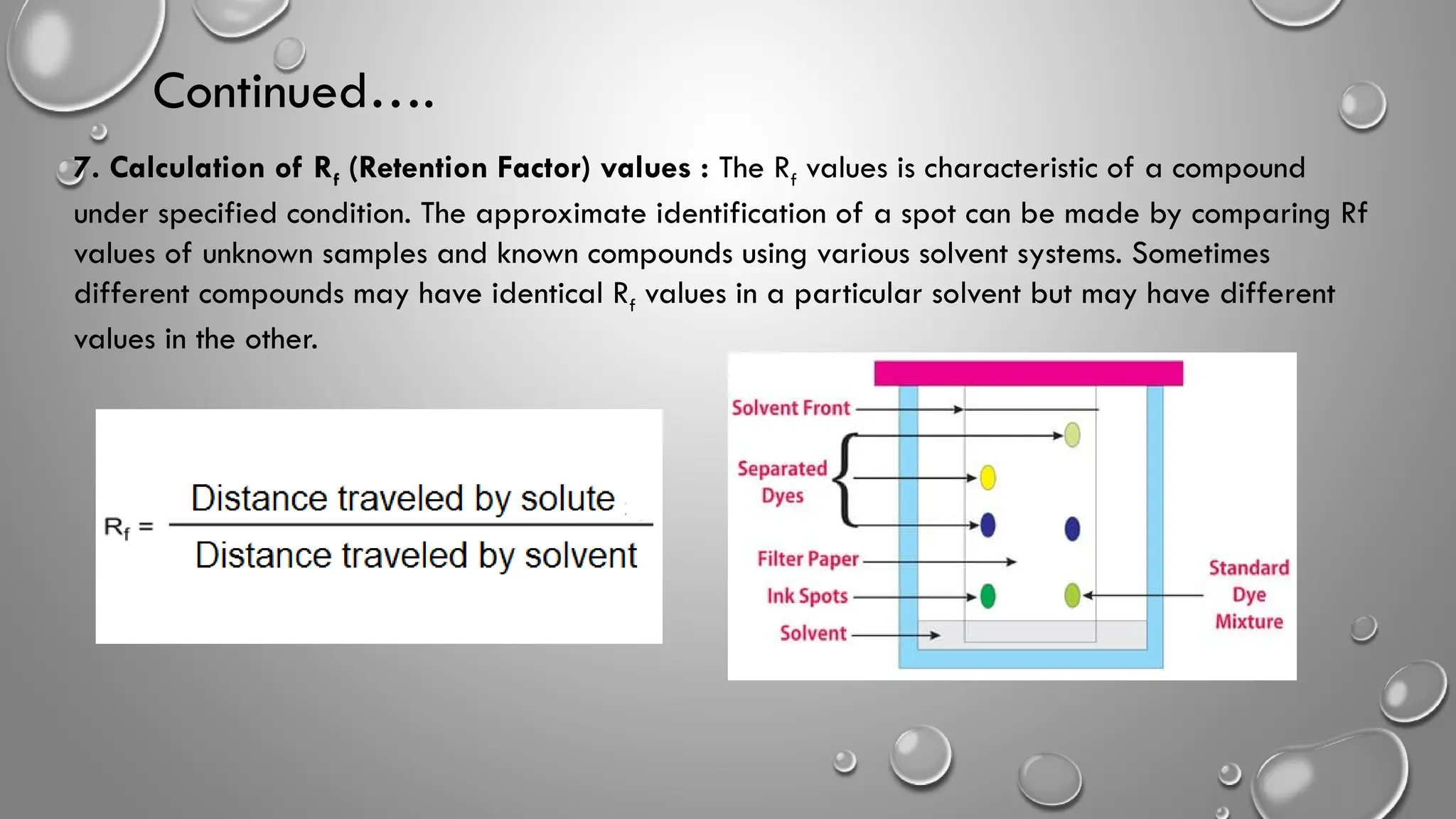
This ppt contains topic mentioned below regarding Paper chromatography
Introduction
History
Principle
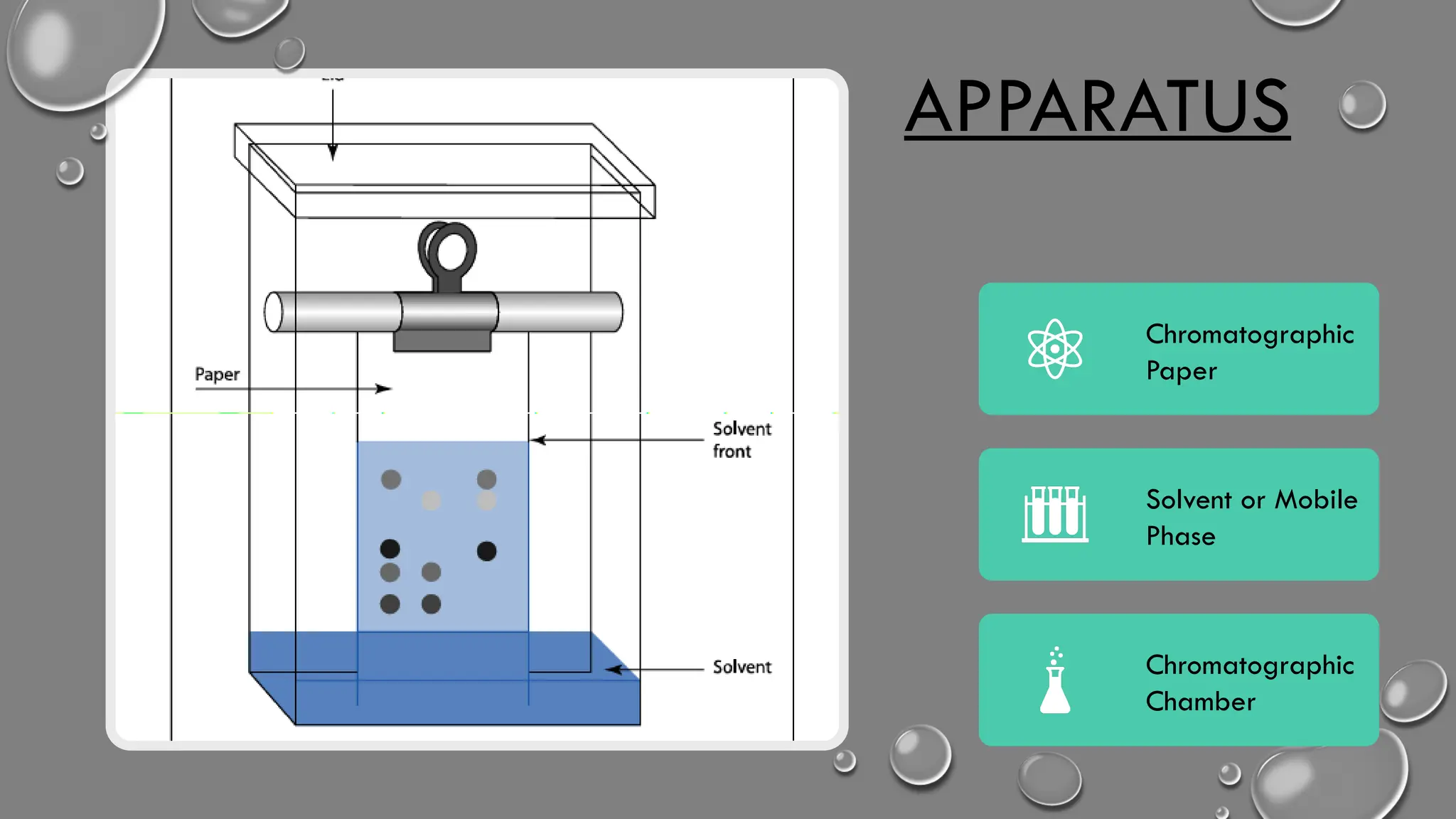
Apparatus
Mechanism of Separation
Types of Chromatography
Factors Affecting the Resolution in Paper Chromatography
Application of paper chromatography