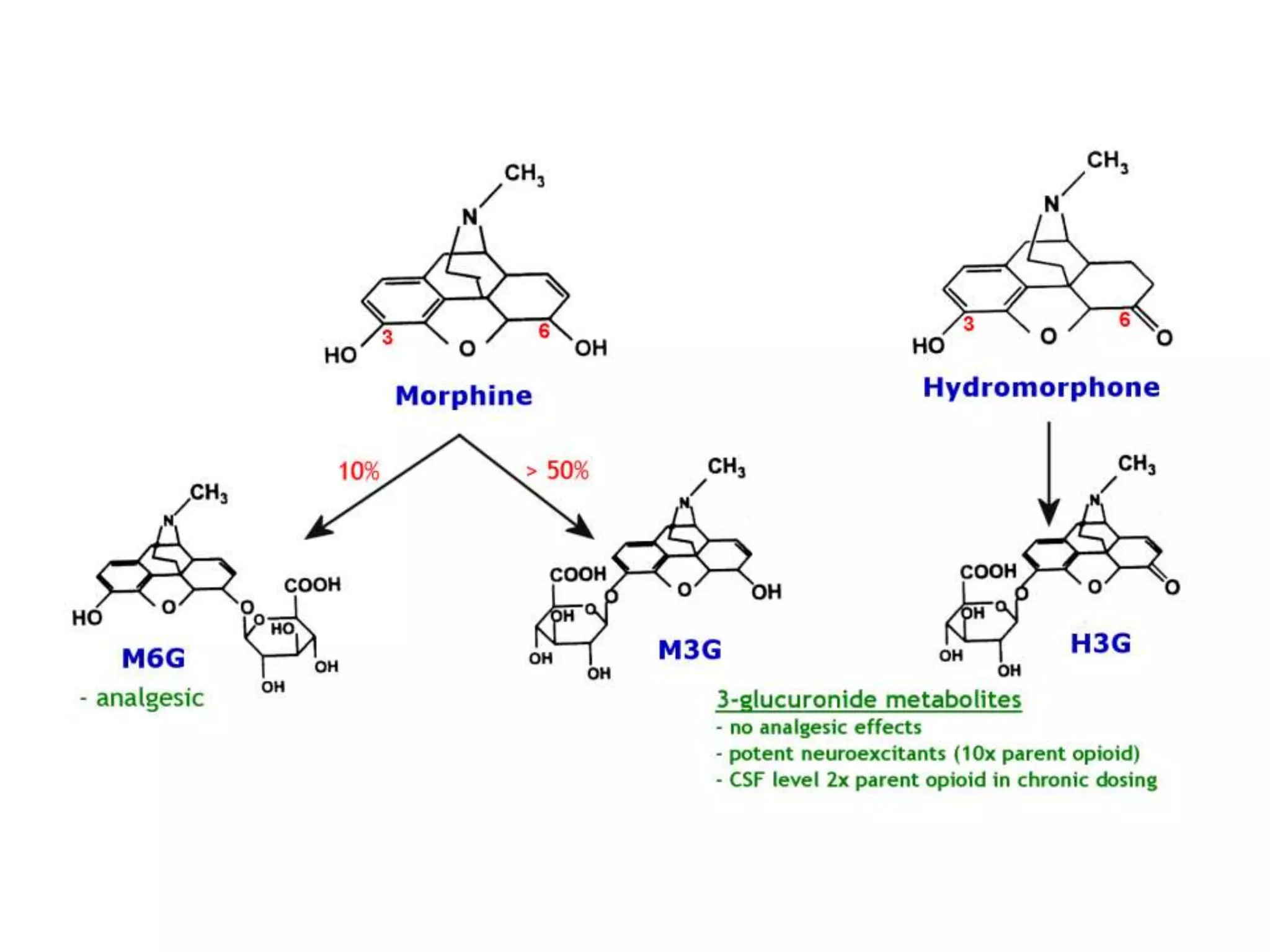
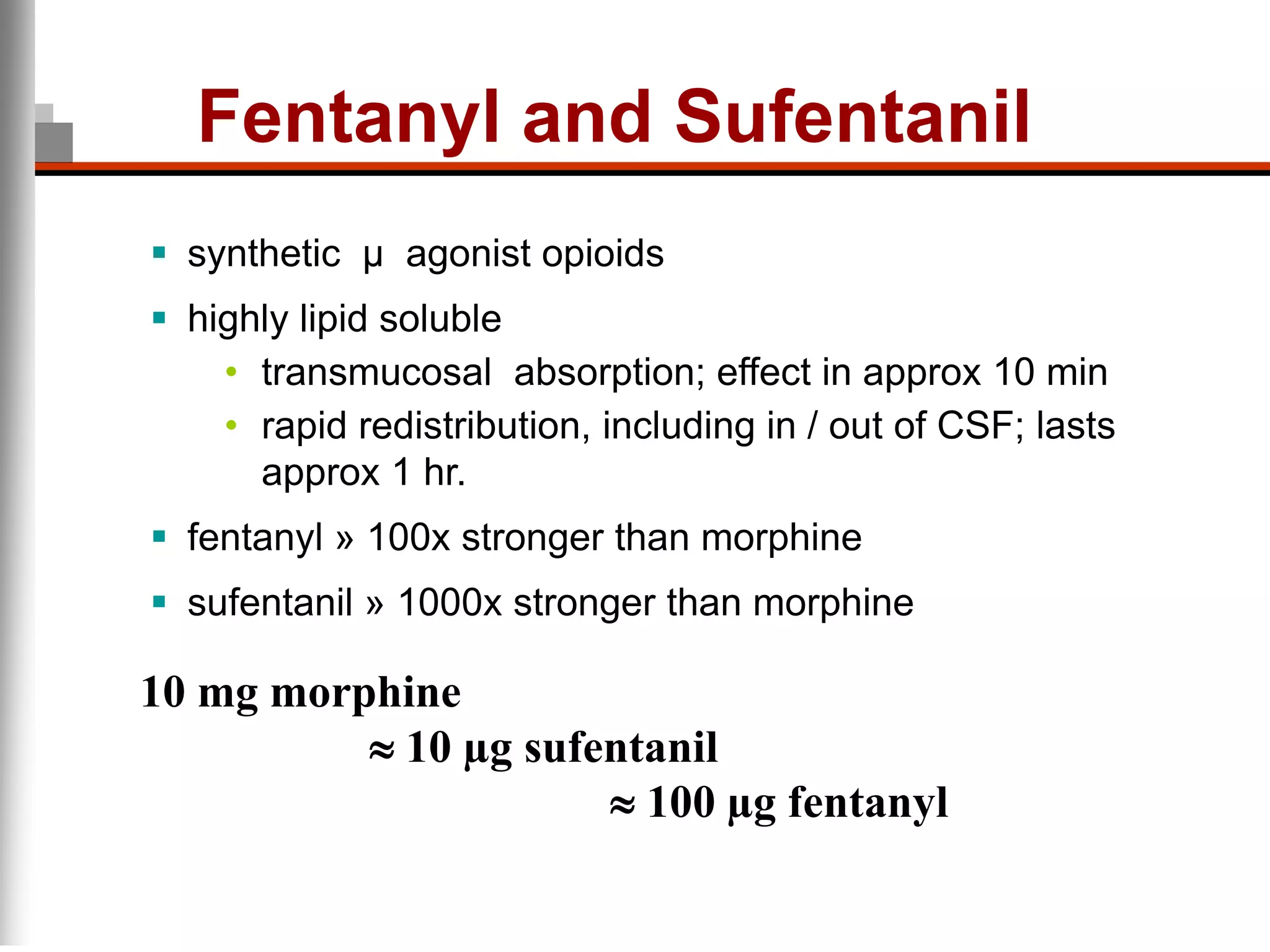
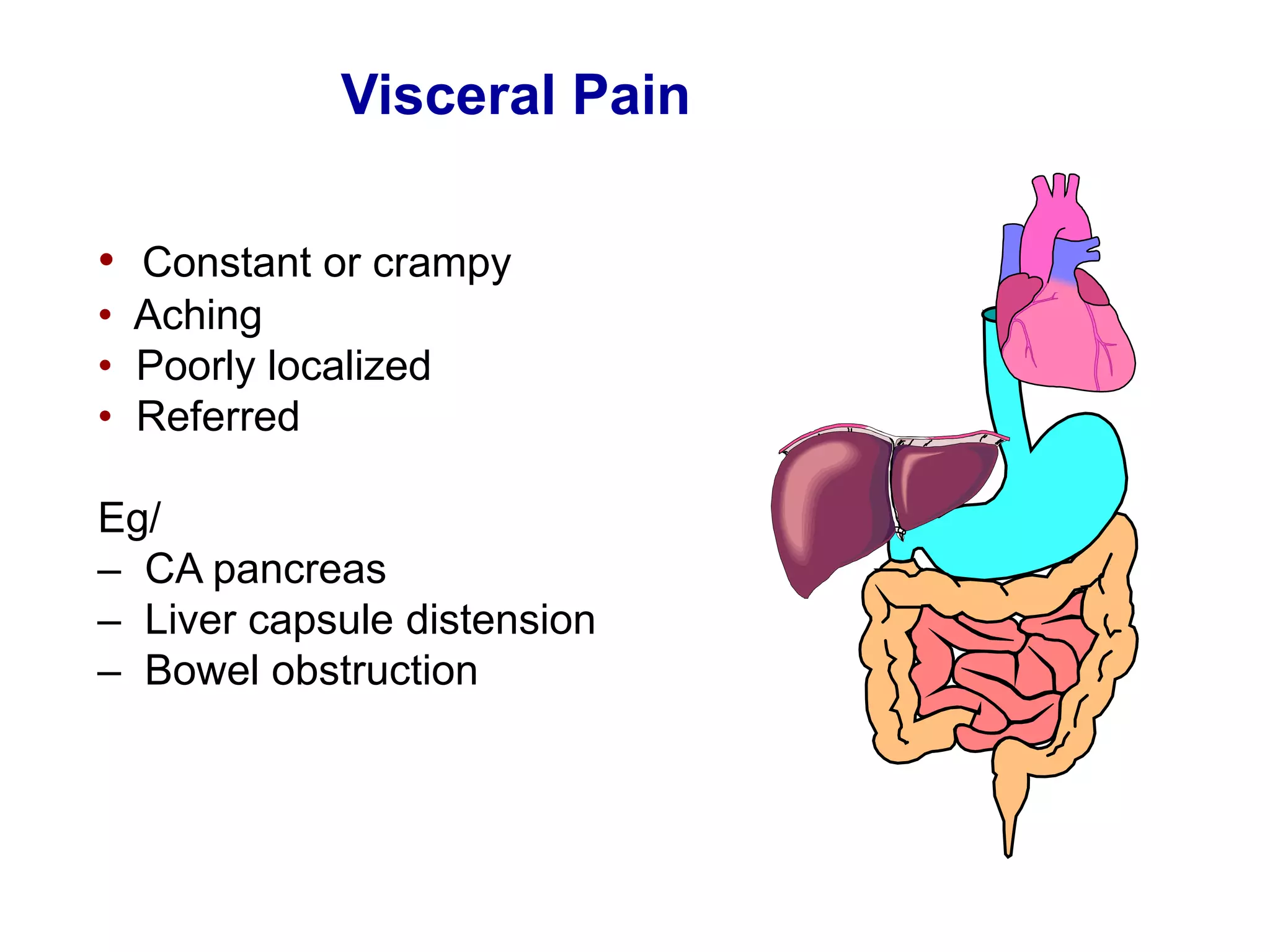
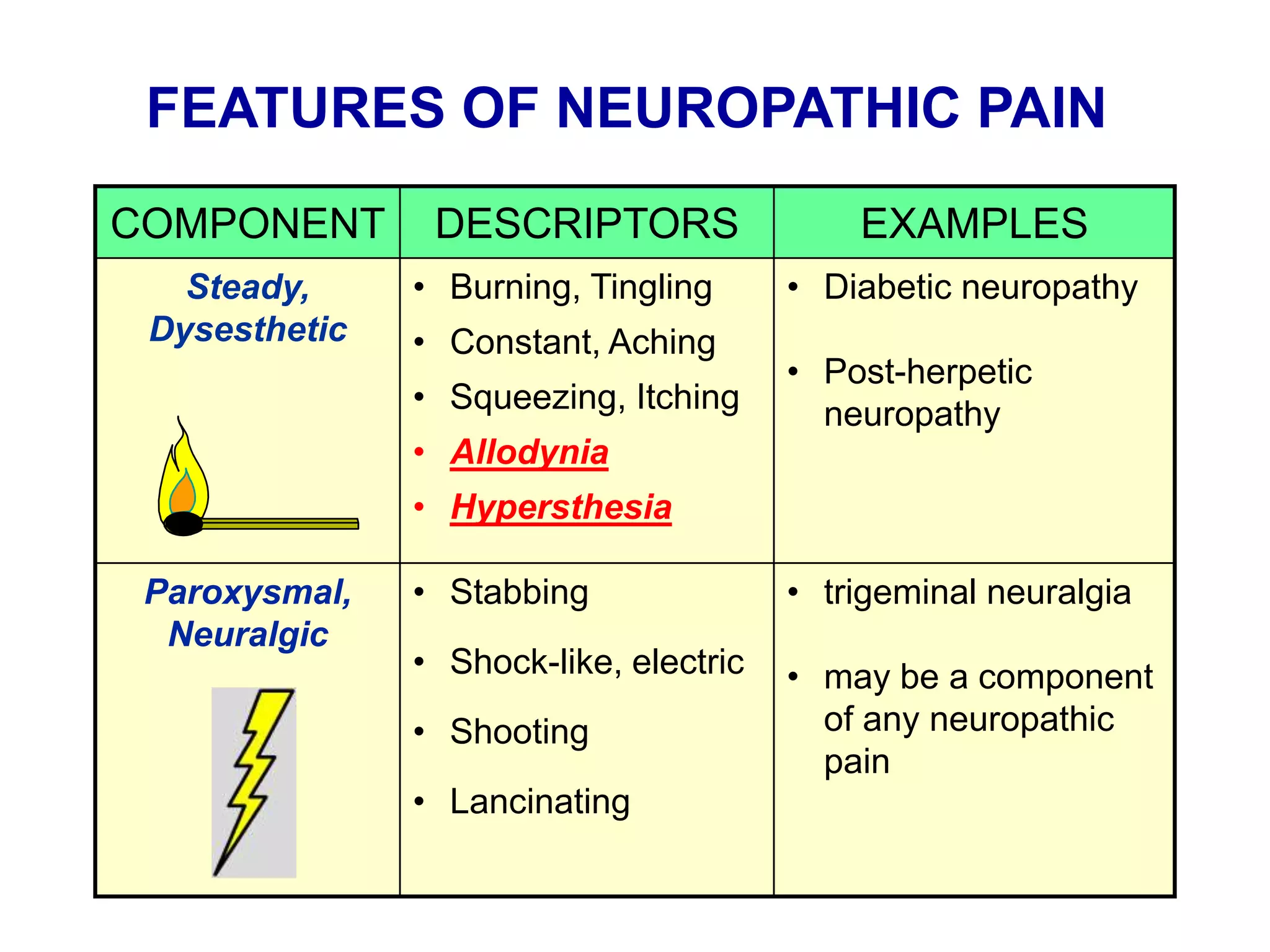
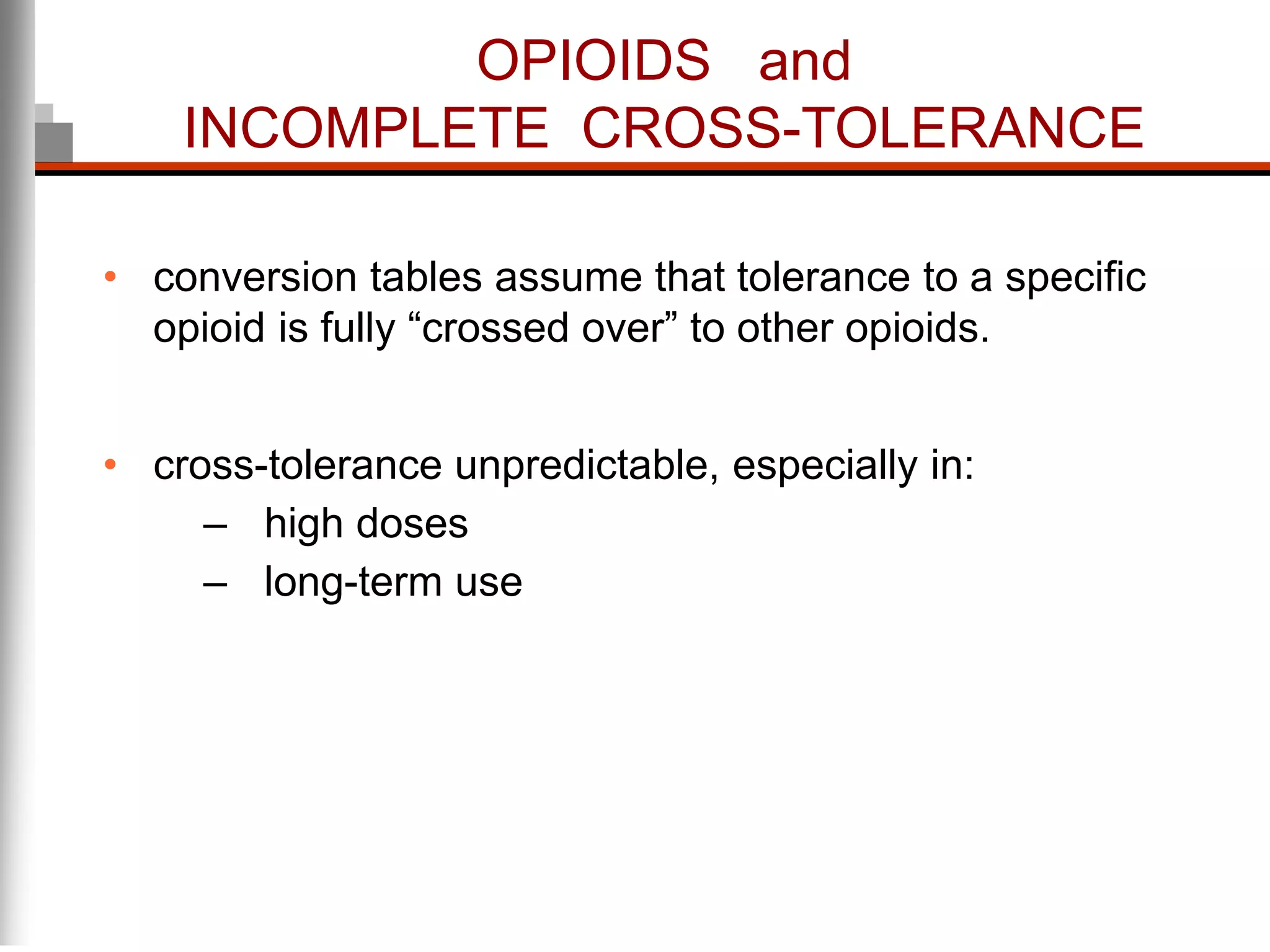
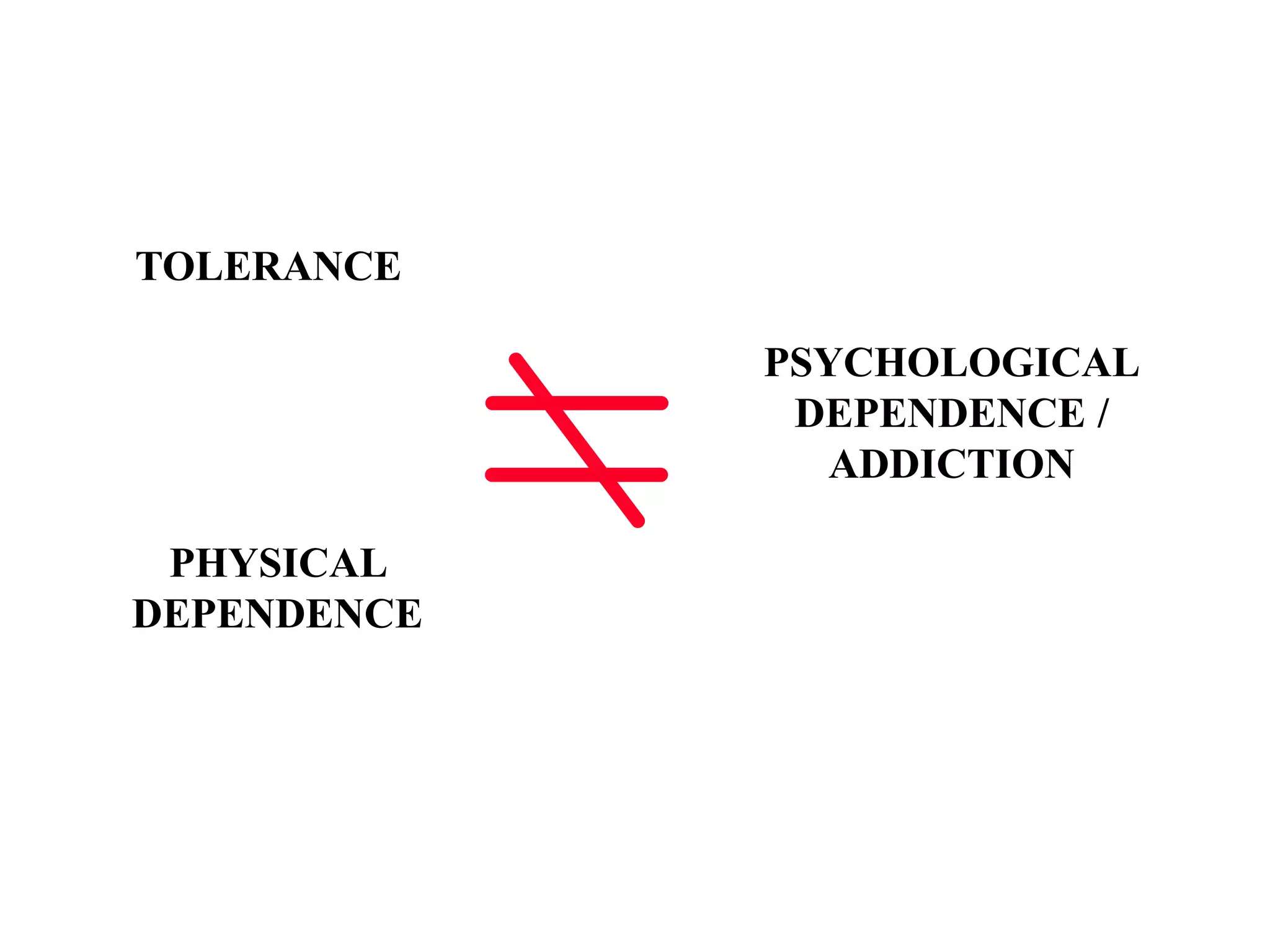
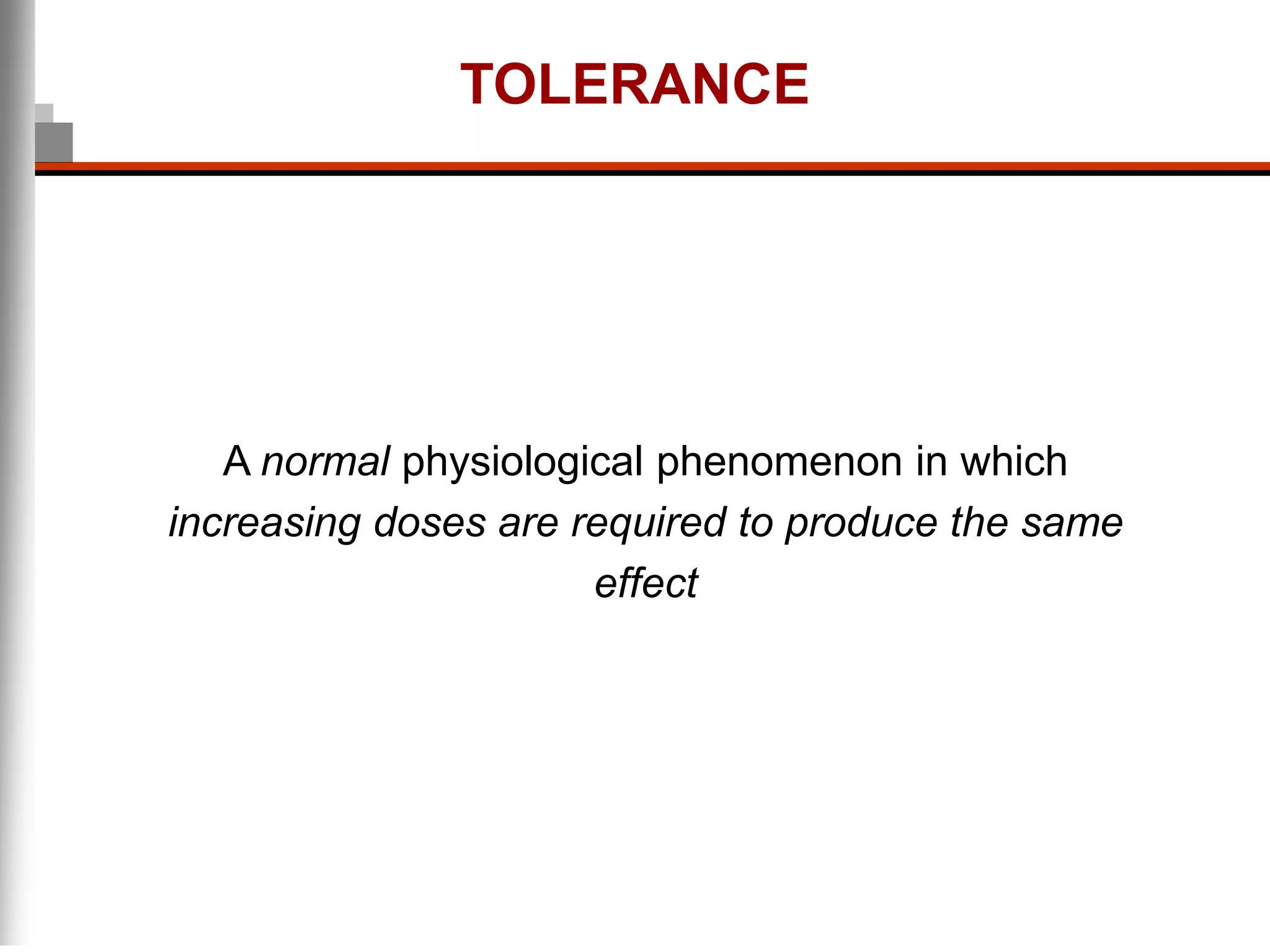
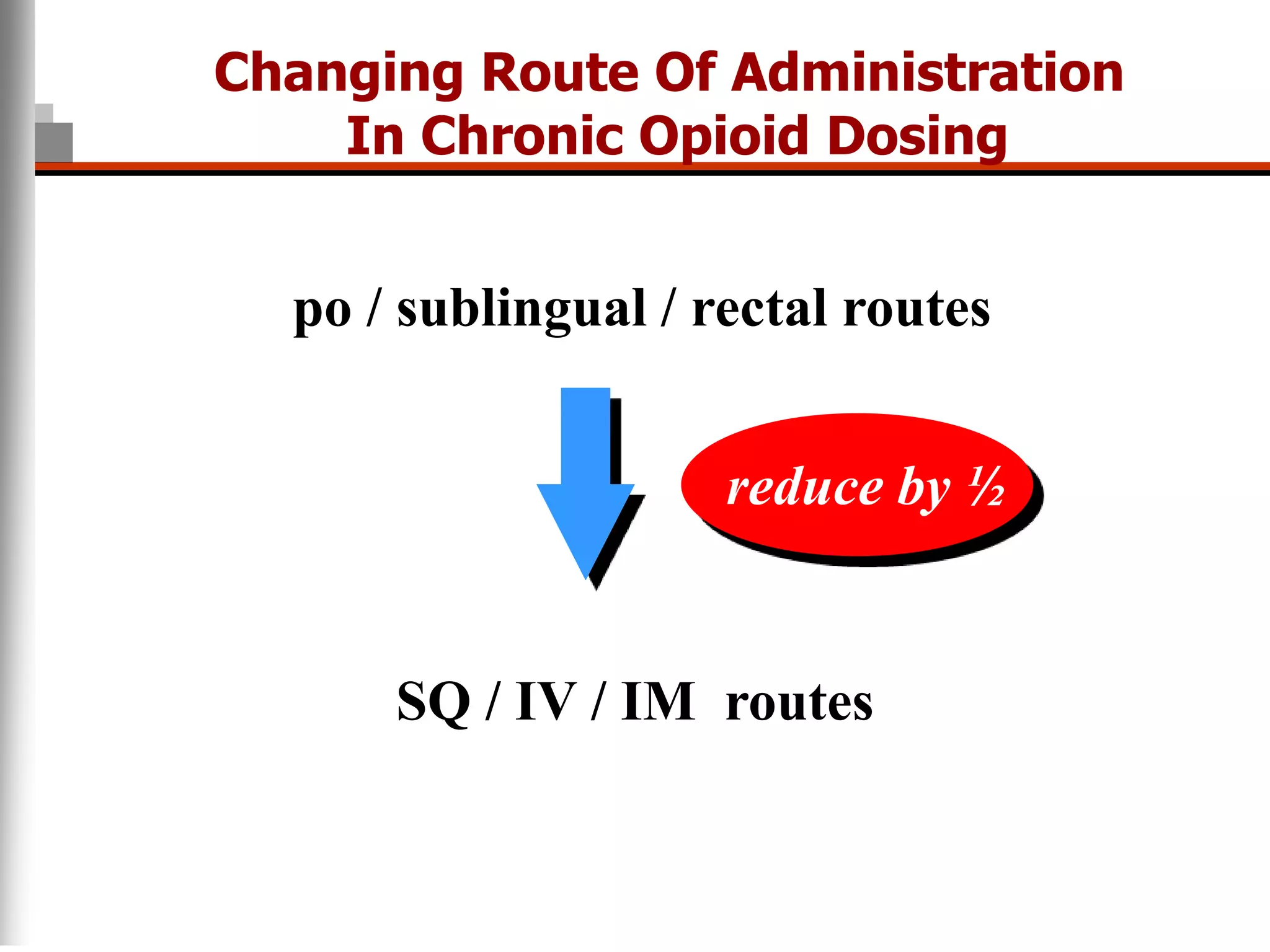
This document discusses pain management in palliative care. It defines types of pain including neuropathic and nociceptive pain. It describes approaches to pain control including thorough assessment, investigations if needed, pharmacological and non-pharmacological treatments, and ongoing reassessment. Types of opioids, routes of administration, switching between opioids, and managing breakthrough pain are covered. Adjuvant analgesics and their uses are also summarized.




























![Opioid Side Effects
Constipation – need proactive laxative use
Nausea/vomiting – consider treating with dopamine
antagonists and/or prokinetics (metoclopramide, domperidone,
prochlorperazine [Stemetil], haloperidol)
Urinary retention
Itch/rash – worse in children; may need low-dose naloxone
infusion. May try antihistamines, however not great success
Dry mouth
Respiratory depression – uncommon when titrated in
response to symptom
Drug interactions
Neurotoxicity (OIN): delirium, myoclonus seizures](https://image.slidesharecdn.com/painpi-230817155424-c4307349/75/pain_pi-ppt-35-2048.jpg)