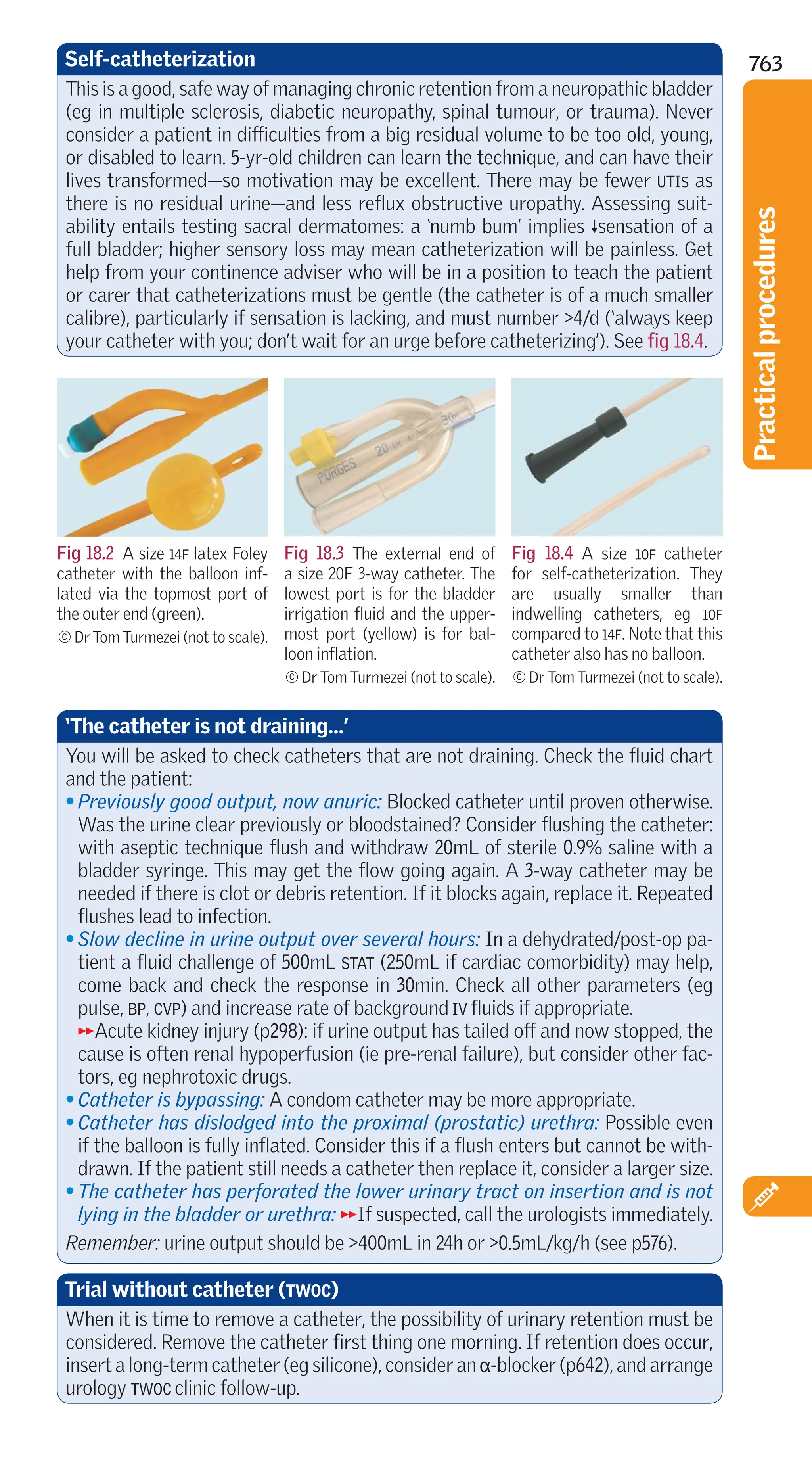
This document provides a summary of the 10th edition of the Oxford Handbook of Clinical Medicine. It begins with prefaces from the original authors and acknowledgments. It then provides an overview of the book's contents which cover major body systems and medical specialties. The book aims to be a concise reference for clinical trainees and physicians that brings together important clinical information in an accessible format. It draws on the experience of multiple physician authors and specialists to provide a comprehensive resource for practicing medicine.














![Thinking
about
medicine
1
The Hippocratic oath ~4th century BC
Iswear by Apollo the physician and Asclepius and Hygieia and Panacea and all the
gods and goddesses, making them my witnesses, that I will fulfil according to my
ability and judgement this oath and this covenant.
To hold him who has taught me this art as equal to my parents and to live my
life in partnership with him, and if he is in need of money to give him a share of
mine, and to regard his offspring as equal to my own brethren and to teach them
this art, if they desire to learn it, without fee and covenant. I will impart it by pre-
cept, by lecture and by all other manner of teaching, not only to my own sons but
also to the sons of him who has taught me, and to disciples bound by covenant and
oath according to the law of physicians, but to none other.
The regimen I shall adopt shall be to the benefit of the patients to the best of
my power and judgement, not for their injury or any wrongful purpose.
Iwill not give a deadly drug to anyone though it be asked of me, nor will I lead
the way in such counsel.1
And likewise I will not give a woman a pessary to pro-
cure abortion.2
But I will keep my life and my art in purity and holiness. I will not
use the knife,3
not even, verily, on sufferers of stone but I will give place to such as
are craftsmen therein.
Whatsoever house I enter, I will enter for the benefit of the sick, refraining
from all voluntary wrongdoing and corruption, especially seduction of male
or female, bond or free.
Whatsoever things I see or hear concerning the life of men, in my attend-
ance on the sick, or even apart from my attendance, which ought not to
be blabbed abroad, I will keep silence on them, counting such things to be as reli-
gious secrets.
If I fulfil this oath and do not violate it, may it be granted to me to enjoy life and
art alike, with good repute for all time to come; but may the contrary befall me
if I transgress and violate my oath.
Paternalistic, irrelevant, inadequate, and possibly plagiarized from the followers of
Pythagoras of Samos; it is argued that the Hippocratic oath has failed to evolve
into anything more than a right of passage for physicians. Is it adequate to address
the scientific, political, social, and economic realities that exist for doctors today?
Certainly, medical training without a fee appears to have been confined to history.
Yet it remains one of the oldest binding documents in history and its principles of
commitment, ethics, justice, professionalism, and confidentiality transcend time.
The absence of autonomy as a fundamental tenet of modern medical care can
be debated. But just as anatomy and physiology have been added to the doctor’s
repertoire since Hippocrates, omissions should not undermine the oath as a para-
digm of self-regulation amongst a group of specialists committed to an ideal. And
do not forget that illness may represent a temporary loss of autonomy caused by
fear, vulnerability, and a subjective weighting of present versus future. It could
be argued that Hippocratic paternalism is, in fact, required to restore autonomy.
Contemporary versions of the oath often fail to make doctors accountable for
keeping to any aspect of the pledge. And beware the oath that is nothing more
than historic ritual without accountability, for then it can be superseded by per-
sonal, political, social, or economic priorities:
‘In Auschwitz, doctors presided over the murder of most of the one million
victims…. [They] did not recall being especially aware in Auschwitz of their
Hippocratic oath, and were not surprisingly, uncomfortable discussing it…The
oath of loyalty to Hitler…was much more real to them.’
Robert Jay Lifton, The Nazi Doctors.
The endurance of the Hippocratic oath
1 This is unlikely to be a commentary on euthanasia (easeful death) as the oath predates the word. Rather,
it is believed to allude to the common practice of using doctors as political assassins.
2 Abortion by oral methods was legal in ancient Greece. The oath cautions only against the use of pessaries
as a potential source of lethal infection.
3 The oath does not disavow surgery, merely asks the physician to cede to others with expertise.](https://image.slidesharecdn.com/q7pxyfctgu9q1j9ffk2p-oxford-handbook-of-clinical-medicine-10th-2017-edition-samansarko-copy-240304020108-0ae7bae7/75/Oxford-HandbooK-LIBRO-SOBRE-MEDICINA-INTERNA-15-2048.jpg)



















![Thinking
about
medicine
21
Number needed to treat
Number needed to treat (NNT) is a useful way of reporting the results of randomized
clinical trials. It is the reciprocal of the absolute risk difference: 1 ÷ ARR.
A large treatment effect means that fewer patients need to receive treatment in
order for one to benefit. It is specific to the chosen comparator (eg placebo or usual
care), the measured outcome (eg death, blood pressure fall), and the duration of
treatment follow-up used in the study. Look carefully at the details of the question
that the NNT is attempting to quantify.
• Advantages: easily calculated, single numerical value for efficacy, can be used to
examine harm (becomes the number needed to harm).
• Disadvantages: confidence intervals are difficult when the differences between
treatments are not significant.
Yes or No? Your tutor asks whether Gobble’s disease is commoner in women or
men. You have no idea, and make a guess. What is the chance of getting it right?
Common sense decrees that you have a 50:50 chance. Sod’s law predicts that
whatever you guess, you will always be wrong. Somewhere between the two is
Damon Runyon’s view that ‘all life is 6 to 5 against’: Will you pick the right answer?
Perhaps, but don’t bet on it!
New or existing disease? Suppose singultus is a rare symptom of Gobble’s dis-
ease (seen in 5% of patients), but that it is a very common symptom of Kobble’s
disease (seen in 90%). If we have a man whom we already know has Gobble’s
disease, who goes on to develop singultus, is it more likely to be due to Kobble’s,
rather than Gobble’s disease? The answer is usually no: it is generally the case that
most symptoms are due to a disease that is already known, and do not imply a
new disease (Occam’s razor, p4). The ‘odds ratio’ makes this clearer, ie the ratio
of [the probability of the symptom, given the known disease] to [the probability
of the symptom due to new disease ≈ the probability of developing the new dis-
ease]. Usually this is vastly in favour of the symptom being due to the old disease,
because of the prior odds of the two diseases. This will work until Kobble’s dis-
ease increases in prevalence so as to increase the odds of a second disease (then
Hickam trumps Occam, p4).
How to play the odds It is distasteful to think that doctors can gamble with
patients’ lives. It is also distasteful to think of serious diseases being ‘missed’, and
invasive procedures being done unnecessarily. Yet we do not have an evidence
base or an experience base which can tell us definitively which cough or lethargy
or sore toe is just ‘one of those things’, and which is the result of undiagnosed
cancer or HIV or osteomyelitis. And so we gamble.
Medicine is not for pessimists—almost anything can be made to seem fatal, so
that a pessimistic doctor would never get any sleep at night due to worry about
the meaning of their patients’ symptoms. Medicine is not for blind optimists either,
who too easily embrace a fool’s paradise of false reassurance. Rather, medicine is
for informed gamblers: gamblers who are happy to use subtle clues to change their
outlook from pessimism to optimism and vice versa. Sometimes the gambling is
scientific, rational, methodical, and reproducible (odds ratio); sometimes it instinc-
tual, due to clinical intuition (vital but ill-defined).
Of course, gambling inevitably results in losses, and in medicine the chips are
not just financial. They betoken the health of your patient, your reputation, and
your confidence. Perhaps the hardest part of medicine is the inevitability of making
mistakes whilst attempting to help (see ‘Being wrong’, p5). But do not worry about
gambling: gambling is your job. If you cannot gamble, you cannot walk the thin line
between successfully addressing health needs, and causing over-medicalization
(p23). But try hard to assemble sufficient evidence to maximize the chances of
being lucky. Lucky gambling is a requisite for successful doctoring and the casino
of medical practice celebrates the card counter. But the cardinal clinical virtue
is courage: without it we would not follow our hunches and take justified risks.
The doctor as a gambler](https://image.slidesharecdn.com/q7pxyfctgu9q1j9ffk2p-oxford-handbook-of-clinical-medicine-10th-2017-edition-samansarko-copy-240304020108-0ae7bae7/75/Oxford-HandbooK-LIBRO-SOBRE-MEDICINA-INTERNA-35-2048.jpg)