This document provides a summary of the table of contents for Kelley's Textbook of Internal Medicine 4th edition published in 2000. It includes over 50 chapters organized into 6 parts covering principles of medical practice, basic mechanisms of health and disease, common primary care issues, clinical evaluation principles, related medical disciplines, and selected medical emergencies. The chapters address topics in cardiology, gastroenterology, nephrology, rheumatology, oncology, infectious diseases, pulmonary medicine, endocrinology and neurology.


























































![associated with the inactivation of transcription.
In certain instances, the removal of a corepressor complex through a ligand–receptor interaction results in the activation of transcription. For example, in the absence
of hormone, the thyroid or retinoic acid receptors are bound to a corepressor complex containing N-CoR or SMRT and associated proteins, some of which have
histone deacetylase activity. The target gene is repressed until the binding of hormone to the thyroid receptor results in the dissociation of this complex, and gene
activation then ensues.
The nuclear actions of steroid hormones are reasonably well defined, but direct actions of these hormones in the cytoplasm and on various organelles and
membranes have also been described. Although steroid hormones affect nuclear mRNA processing, mRNA degradation rates, and post-translational processing, most
evidence suggests that these hormones exert their predominant effect on the transcription of specific genes.
MECHANISM OF ACTION OF GROUP II POLYPEPTIDE HORMONES
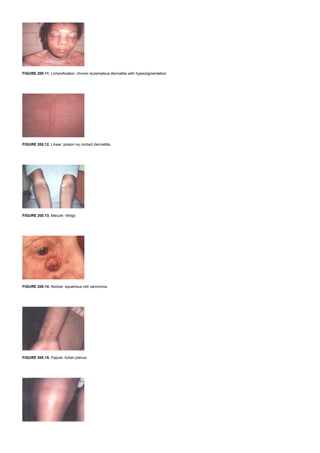
The majority of hormones are water-soluble, have no transport proteins (with the exception of insulin-like growth factors IGF-I and IGF-II), and therefore have a short
plasma half-life. They initiate a response by binding to a receptor located in the plasma membrane, an interaction that results in the generation of an intracellular
signal or messenger that then mediates the action of the hormone (Table 7.1 and Table 7.2). The mechanisms of action of this group of hormones can be discussed
best in terms of their intracellular messengers.
CYCLIC AMP AS THE SECOND MESSENGER
Cyclic AMP, a ubiquitous nucleotide derived from ATP through the action of the enzyme adenylate cyclase (located on the inner surface of the plasma membrane),
plays a crucial role in the action of several hormones. The intracellular level of cAMP is increased or decreased by various hormones that activate or inactivate
adenylate cyclase (Table 7.3). This process is mediated by at least two types of GTP-dependent regulatory protein complexes, designated G s (stimulatory) and Gi
(inhibitory). Hormones that bind to receptors coupled to G s activate adenylate cyclase and increase cAMP production, and hormones that bind to receptors coupled to
Gi decrease cAMP production. Gq is a pertussis-resistant G protein that can activate phosphoinositi- dase C.
TABLE 7.3. SUBCLASSIFICATION OF GROUP II HORMONES FOR WHICH THE SECOND MESSENGER IS CAMP
The components of the cyclase system have been purified, revealing a large family of G proteins. The G-protein complexes are heterotrimers consisting of a, b, and g
peptide chain subunits. There are at least 16 a subunits, 6 b subunits, 12 g subunits, and 8 different adenylate cyclases, so that many different combinations are
possible. The a subunit, active when bound to GTP, contains an intrinsic GTPase activity that hydrolyzes GTP to GDP. The a subunit is inactive when bound to GDP,
providing a tightly regulated on–off mechanism. The a subunits, in addition to regulating adenylate cyclase activity, can also regulate ion channel activity. Some forms
of as stimulate K+
channels and inactivate Ca2+
channels, and some ai isoforms inhibit K+
channels and activate Ca2+
channels. The Gq heterocomplexes activate
phospholipase C. The bg-subunit complexes have also been shown to activate K+
channels and phospholipase C. In addition to hormone-regulated metabolic
processes, such as gluconeogenesis, lipolysis, and glycogenolysis, G proteins are involved in many other important and diverse biologic processes, including
neuronal activity, heart rate, blood pressure control, muscle contraction, cell replication, vision, and smell.
In eukaryotic cells, cAMP activates a protein kinase that is a heterotetrameric molecule consisting of two regulatory subunits (R) and two catalytic subunits (C). cAMP
binds to the regulatory subunits and results in the following reaction: 4 cAMP + R 2C2 R2× (4 cAMP) + 2C. The R2C2 complex has no enzymatic activity, but the
binding of cAMP by R dissociates R from C, thereby activating the latter. The active C subunit catalyzes the transfer of the g-phosphate of adenosine triphosphate
(ATP) in an Mg2+
-dependent reaction to a serine or threonine residue in a variety of proteins, resulting in altered activity of the protein.
Reactions caused by hormones in this class can be terminated in several ways, including the hydrolysis of cAMP by phosphodiesterases. The presence of these
hydrolytic enzymes ensures a rapid turnover of the signal (cAMP); termination of the biologic process is rapid after the hormonal stimulus is removed. The cAMP
phosphodiesterases are themselves subject to regulation by hormones and by intracellular messengers such as calcium, probably acting through calmodulin.
Inhibitors of phosphodiesterase, most notably xanthine derivatives (e.g., caffeine, theophylline), increase intracellular cAMP and mimic or prolong the actions of
hormones. Another means of controlling hormone action is the regulation of the protein dephosphorylation reaction. The phosphoprotein phosphatases are
themselves subject to regulation by phosphorylation–dephosphorylation reactions and by a variety of other mechanisms, often as a consequence of the action of a
hormone.
CYCLIC GMP AS THE SECOND MESSENGER
Cyclic GMP is made from GTP by the enzyme guanylate cyclase, which exists in soluble and membrane-bound forms. Each of these isozymes has unique kinetic,
physiochemical, and antigenic properties. The atriopeptins, a family of peptides produced in cardiac atrial tissue, cause natriuresis, diuresis, vasodilatation, and
inhibition of aldosterone secretion. These peptides (e.g., atrial natriuretic factor) bind to and activate the membrane-bound form of guanylate cyclase. This action
increases the concentration of cGMP, by as much as 50-fold in some cases, which is thought to mediate the effects of these peptides.
Other evidence links cGMP to vasodilatation. A series of compounds, including nitroprusside, nitroglycerin, sodium nitrite, and sodium azide, cause smooth muscle
relaxation and are potent vasodilators. These agents activate nitric oxide synthase, which catalyzes the formation of nitric oxide. Nitric oxide activates guanylate
cyclase, which produces cGMP. The increased cGMP concentration activates cGMP-dependent protein kinase, which phosphorylates several smooth muscle
proteins, including the myosin light chain. Presumably, this mechanism is involved in relaxation of smooth muscle and vasodilatation. This reaction is terminated by a
specific cGMP-dependent phosphodiesterase, PDE type 5. [PDE5 is the target of sildenafil (Viagra), and this accounts for the smooth muscle relaxation effect of this
drug and its activity in erectile dysfunction.]
CALCIUM AND PHOSPHATIDYLINOSITIDES AS SECOND MESSENGERS
Ionized calcium is an important regulator of a variety of cellular processes, including muscle contraction, stimulus–secretion coupling, the blood clotting cascade,
enzyme activity, and membrane excitability. It also is an intracellular messenger of hormone action.
A role for ionized calcium in hormone action is suggested by several observations. First, the hormone effect is blunted when tested in Ca 2+
-free media or when
intracellular calcium is depleted. Second, the effect can be mimicked by agents that increase cytosolic Ca 2+
, such as the Ca2+
ionophore A23187. Third, the hormonal
effect involves changes of cellular calcium movement. These processes have been studied in some detail in cells of the pituitary, smooth muscle, platelets, and
salivary gland, but most is known about how vasopressin and a-adrenergic catecholamines regulate glycogen metabolism in liver.](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-67-320.jpg)










![Because of the imprecision of valance and equivalence for this electrolyte, serum phosphorus concentrations are routinely reported in mass units (milligrams per
deciliter).
OSMOLALITY
The osmolality of a given body fluid is determined by the concentration of the circulating dissociated ionic and nonionic particles contained in that fluid. In the
extracellular fluid volume, since sodium makes up more than 95% of the circulating cationic ionized particles, and glucose and urea account for virtually all of the
normally occurring circulating nonionic particles, the total plasma osmolality can be easily approximated by the formula: plasma osmolality = 2× [plasma sodium
concentration] + glucose concentration (mg %) ÷ 18 + BUN ÷ 2.8. This value is referred to as the calculated plasma osmolality and, as mentioned above, equals the
osmolality of the entire ECF and the osmolality of the ICF as well. The pathology laboratory confirms the measurement of plasma osmolality by use of a technique that
measures the concentration of osmotically active particles through its effect on depressing the freezing point of any given solution. In general, the difference between
the calculated plasma osmolality and the measured plasma osmolality is less than 5 to 10 mOsm per L.
While determination of the plasma osmolality is important, it is vital to recognize that not all particles contributing to the plasma osmolality are osmotically effective.
The most significant exception is urea. Because of its permeability across cell membranes, intracellular and extracellular urea concentrations are equal; in effect, the
osmotic pressure that they would generate across these fluid compartments “cancels itself out.” Hence, clinicians are much better served by calculating the effective
plasma osmolality by the formula noted above with the urea term removed. Effective osmolality is also known as tonicity, a term that many find useful in distinguishing
between total osmolality and effective osmolality.
TONICITY AND CELL VOLUME
As noted above, when water is added to the body it is distributed between the two major body fluid compartments such that at steady state two-thirds is located in the
ICF and one-third in the ECF. Similarly, a pure water deficit is distributed such that at steady state two-thirds of the net loss is derived from the ICF and one-third from
the ECF. Because the gain or loss of pure water is shared proportionately by the two major body fluid compartments, it does not alter the relative volumes of these two
compartments.
If an effective solute (e.g., sodium, glucose) is added to the ECF, the ECF tonicity increases and water moves out of the cells. The ECF volume increases at the
expense of the ICF volume until the tonicity of the two fluid compartments is equalized. Conversely, if an effective solute is removed from the ECF, the ECF tonicity
decreases and water moves into the cells. The ICF volume increases at the expense of ECF volume until the tonicity of the two fluid compartments is equalized. In
contrast, if an ineffective solute (e.g., urea) is added to or removed from the ECF, the ECF tonicity does not change and cell volume remains constant.
Changes in body fluid tonicity are associated with characteristic alterations in cell volume. Hypertonicity leads to ICF contraction (cell shrinkage or dehydration), while
hypotonicity leads to ICF expansion (cell swelling or edema). The major clinical features of disorders of water homeostasis are largely attributable to changes in cell
volume.
CLINICAL IMPLICATIONS
As a consequence of these physiologic parameters it becomes relatively easy to assess the effect of intravenous replacement solutions on patients with various body
fluid deficits. The administration of 5% dextrose in water, while effectively addressing a water deficit, does little to expand plasma volume in particular. One liter of 5%
dextrose in water, for example, in the absence of diabetes, will distribute 667 mL to the ICF and 333 mL to the ECF. Three-fourths of that fluid will be retained in the
interstitial compartment, leaving approximately 85 mL of the original liter to distribute to the plasma volume, a poor plasma expander indeed.
Administration of Ringer's lactate, normal saline, or any other isonatric sodium containing solution is far more effective in plasma volume expansion. One liter of
normal saline will remain entirely in the extracellular fluid (sodium being limited from entry to the intracellular compartment by active extrusion from virtually all cells).
Of the liter retained in the ECF compartment, 250 mL distributes to the plasma volume—a far better effect. For the ultimate volume expander, plasma infusion is
theoretically ideal. An entire liter of infused plasma (because of its iso-osmotic and iso-oncotic pressures) remains in the plasma volume and would be most effective
in emergency plasma volume expansion.
The converse physiologic circumstance to that noted above is also true. Water loss is well tolerated with regard to hemodynamics; salt loss (with its accompanying
water) is more significant; and plasma loss can be fatal.
SERUM SODIUM CONCENTRATION
Sodium salts make up more than 95% of ECF effective solutes, and in most circumstances, the serum sodium concentration ( SNa) accurately reflects body fluid
tonicity. Because the symptoms and signs of abnormal body fluid tonicity are generally nonspecific, disorders of water homeostasis are often detected clinically by the
presence of an abnormal SNa. The features of altered body fluid tonicity relate to changes in tonicity and not to associated changes in SNa. Consequently, tonicity and
SNa need not always change concordantly. Although hypernatremia always implies hypertonicity, the converse is not always true; hyponatremia does not always imply
hypotonicity.
Hypertonicity can occur in the absence of hypernatremia when an effective solute other than sodium (e.g., glucose) is present in excessive amounts in the ECF. The
osmotic pressure exerted by the nonsodium solute leads to redistribution of water from the ICF to the ECF and consequently leads to hyponatremia and intracellular
volume depletion. Hyperglycemic hypertonicity is common in patients with uncontrolled diabetes mellitus.
Hyponatremia can occur in the absence of hypotonicity if an effective solute other than sodium is present in significant quantity in the ECF (hypertonic hyponatremia)
or when large amounts of lipids or proteins are present in the plasma (isotonic hyponatremia). The latter circumstance, also known as pseudohyponatremia, is
discussed in Chapter 144.
WATER BALANCE
Water balance in a normal person is maintained through a series of hemodynamic, hormonal, and molecular mechanisms. Balance is so finely tuned that neither
water retention nor water loss will occur despite changes in fluid intake of 10-fold or greater (e.g., 1 L to 10 L per day). One of the characteristics of chronic renal
disease (see Chapter 133 and Chapter 141) is that this broad range over which water balance can be achieved is narrowed significantly because the homeostatic
mechanisms have themselves been compromised by the process, causing injury to the kidney.
The following sequence of events occurs after the ingestion of water in a normal individual ( Fig. 9.1). Plasma osmolality is transiently diluted and the effective
osmolality falls; extracellular fluid volume is expanded, albeit minimally. This expansion results in a minimal, clinically undetectable increase in glomerular filtration
rate and delivery of more sodium to the proximal tubule of the kidney (see below). As a consequence of the dilution of effective plasma osmolality, antidiuretic
hormone (ADH; also known as vasopressin) production and release is inhibited at the level of the hypothalamus. This inhibition lowers the level of circulating ADH
and decreases its effects on the distal portions of the nephron: the distal cortical tubule, the collecting tubule, and the collecting duct. Also, the increase in
extracellular fluid volume and increased perfusion of the hypothalamus sends a signal of hypervolemia to a variety of volume receptors throughout the body, which
further inhibit ADH synthesis and release. The increased sodium delivered to the proximal tubule is for the most part reabsorbed, but a greater absolute amount of the
filtered sodium travels down to the prime diluting site in the ascending limb of the loop of Henle, where chloride (with sodium following passively) is removed and
water stays behind. This segment of the nephron is unalterably impermeable to water. The increased volume of hypotonic fluid enters the cortical distal tubule,
collecting tubule, and collecting duct (these structures are impermeable to water due to the absence of ADH), and increased urine volume accompanied by increased
excretion of water results. Subsequently, the plasma osmolality, extracellular fluid volume, and glomerular filtration rate return to normal.](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-78-320.jpg)




















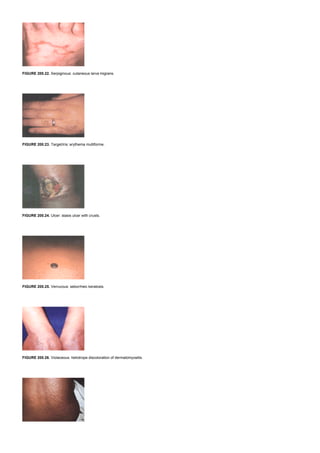
![FIGURE 12.5. Pathways of HDL metabolism. Solid lines trace the conversion of lipoprotein particles. Broken lines trace the transfer of free cholesterol or cholesteryl
ester between different lipoprotein classes or between lipoprotein and cells. ABC1, ATP-binding cassette protein 1; CETP, cholesteryl ester transfer protein; HL,
hepatic lipase; LCAT, lecithin:cholesterol acyltransferase; SR-BI, scavenger receptor class BI.
High-density lipoproteins are believed to participate in a process termed “reverse cholesterol transport” in which excess peripheral cholesterol is acquired by HDLs
and returned to the liver for excretion into the bile ( Fig. 12.5). Apolipoprotein A-I, the major HDL apolipoprotein, is synthesized by both the intestine and the liver.
Nascent HDLs are thought to be discoidal particles that acquire unesterified cholesterol from peripheral tissues, a process facilitated by a cellular protein called
ATP-binding cassette protein 1 (ABC1). Unesterified cholesterol is esterified on HDLs by the enzyme lecithin:cholesterol acyltransferase (LCAT), a plasma enzyme
associated with HDLs. As nascent HDLs generate more cholesteryl ester, they evolve into spherical particles, which enlarge further as they acquire additional lipid
and protein constituents from chylomicrons and VLDLs when the triglyceride core of these lipoproteins is hydrolyzed. The HDL cholesteryl esters can be transferred to
the liver through at least two pathways. First, HDL cholesteryl esters can be transferred to apoB-containing lipoproteins in exchange for triglyceride through the action
of cholesteryl ester transfer protein (CETP). Some of these apoB-containing lipoproteins are then removed from the plasma by the liver via the LDL receptor or LRP.
Triglyceride-enriched HDL is a substrate for hepatic lipase, which hydrolyzes HDL triglycerides and phospholipids and generates smaller HDL particles. Second, HDL
cholesteryl esters can be taken up directly by the liver in a process termed selective cholesterol uptake. This process is mediated by scavenger receptor class BI
(SR-BI), a cell surface receptor that facilitates uptake of HDL cholesteryl ester but not of the entire lipoprotein particle. Thus, the metabolism of HDL cholesteryl ester
and HDL apoA-I are dissociated. Eventually, apoA-I is removed from the circulation by a process that is not well understood but involves both the kidneys and the
liver.
ApoA-II is the second most abundant HDL apolipoprotein. Unlike apoA-I, apoA-II is synthesized only by the liver, not by the intestine. Apolipoprotein A-II is found on
approximately two-thirds of all HDL particles. This results in two major classes of HDL particles: one containing apoA-I and apoA-II and the other containing apoA-I
without apoA-II. These two classes of HDL particles may have different physiologic roles and metabolic pathways, but their significance for human pathophysiology is
uncertain.
LIPOPROTEIN(A) (FIG. 12.6)
FIGURE 12.6. Pathways of lipoprotein(a) metabolism. Apolipoprotein(a) is secreted by the hepatocyte independently of LDL and subsequently binds to LDL or a
precursor to form Lp(a). HL, hepatic lipase; LPL, lipoprotein lipase.
Lipoprotein(a), or Lp(a), is another lipoprotein found to a variable extent in human plasma. It is related in structure to LDL in that it contains apoB-100 and a core of
cholesteryl ester. Lipoprotein(a) is distinguished structurally from LDL by the presence of a unique protein, apolipoprotein(a) [apo(a)], which is linked by a single
disulfide bond to the apoB. Apolipoprotein(a) bears a strong resemblance to plasminogen and varies substantially in its molecular weight among different persons. It
is synthesized by the liver, and the association of apo(a) with apoB may occur after apo(a) is secreted into the plasma. Once intact, the metabolism of Lp(a) is distinct
from that of LDL. Lipoprotein(a) generally has a longer half-life than that of LDL and is not removed from the plasma by the LDL receptor. However, the site and
mechanism of its catabolism are unknown. Lipoprotein(a) is generally considered to be an independent risk factor for atherosclerotic vascular disease.
INTRACELLULAR CHOLESTEROL METABOLISM (FIG. 12.7)
FIGURE 12.7. Intracellular cholesterol metabolism. Low-density lipoprotein (LDL) is targeted to lysosomes by the LDL receptor and LDL cholesteryl esters are
hydrolyzed to free cholesterol by the lysosomal acid lipase. The Niemann-Pick C gene 1 is required for transfer of lysosomal free cholesterol to the cytoplasmic pool.
Free cholesterol can then be esterified to cholesteryl esters by acyl coenzyme A:cholesterol acyltransferase and stored as lipid droplets or be released to acceptor
particles such as HDL. Free cholesterol within the cell down-regulates the expression of genes such as b-hydroxy-b-methylglutaryl-coenzyme A reductase and the
LDL receptor. ACAT, acyl coenzyme A:cholesterol acyltransferase; CE, cholesteryl ester; FC, free cholesterol; NPC1, Niemann-Pick C gene 1.
Cells require cholesterol for normal function, and they obtain it from two sources: biosynthesis from acetyl CoA and uptake of lipoprotein cholesterol from the
interstitial fluid. Most cells can take up cholesterol in the form of LDL through the LDL receptor, with the interaction mediated by apoB-100. The LDL receptor is a
glycoprotein that, after insertion into the plasma membrane, migrates laterally to coated pits, regions specialized for the rapid uptake of macromolecules such as LDL.
After binding to the receptor, LDL is internalized in endosome and dissociated from the receptor, which then recycles to the cell surface to take in more LDL. The](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-99-320.jpg)











![CHAPTER 13: PATHOGENESIS OF ATHEROSCLEROSIS
Kelley’s Textbook of Internal Medicine
CHAPTER 13: PATHOGENESIS OF ATHEROSCLEROSIS
ALAN M. FOGELMAN, FRANKLIN L. MURPHY AND PETER A. EDWARDS
Lesion Development
Genetic Predisposition to Atherosclerosis
Treatment Strategies
LESION DEVELOPMENT
The earliest morphologic change in the development of most atherosclerotic lesions is the appearance of mononuclear cells at sites that are destined to become
lesions. At least 90% of these cells are blood monocytes. They diapedese between endothelial cells and come to rest in the subendothelial space. Here they convert
to macrophages and accumulate lipid droplets rich in cholesteryl esters. Because of the high lipid content, their cytoplasm has a foamy appearance in histologic
sections—hence the name foam cells. The number of these cells markedly increases in the subendothelial space and deforms the overlying endothelium. With time
the endothelial monolayer may develop microscopic separations between cells that expose the underlying foam cells and extracellular matrix. These exposed areas
serve as sites of platelet adherence, aggregation, and release.
Mitogenic substances from platelets (e.g., platelet-derived growth factor), endothelial cells, and monocytes stimulate the proliferation of smooth muscle cells that
migrate into the sub- endothelial space. As cells die, their cytoplasmic contents are released, and together with plasma-derived lipoproteins the lipid-rich extracellular
matrix increases in size. The cellular and extracellular components are continuously replaced in the subendothelial space, and some foam cells migrate back into the
bloodstream. The expanding lesion pushes out from the subendothelial space to the adventitia. In some species, including humans, a compensatory hypertrophy of
the artery wall allows further expansion toward the adventitia. If the process continues unabated, however, eventually outward expansion is no longer possible and
encroachment on the lumen begins.
The spatial nature of this process is clinically important because it means that normal angiograms may be seen in subjects with extensive lesions that have not yet
produced luminal narrowing. It is not rare to see patients with an essentially normal angiogram a year before an angiogram showing extensive three-vessel luminal
narrowing. Presumably, the process was present extensively at the time of the first angiogram but was confined to the artery wall. In some cases, the episode of chest
pain that led to the first angiogram was induced by vasospasm at the site of such an intramural lesion.
Evidence suggests that the atherosclerotic process impairs the normal ability of the artery wall to generate endothelial-derived relaxing factors. Consequently, instead
of the normal response (dilatation) to various stimuli (e.g., exercise), the atherosclerotic artery contracts. Subsequently, in the course of only 1 or 2 years, such
intramural lesions may expand into the lumen, particularly in hyperlipidemic subjects.
As the lesion develops, its character can change. Many early lesions contain only macrophage foam cells. These are called fatty streaks and do not cause luminal
narrowing. Many fatty streaks do not progress. At predictable sites in the arterial tree, however, these lesions can develop a fibrous cap, and prominent smooth
muscle proliferation may occur. Advanced lesions contain a necrotic lipid core and proteins that are associated with calcification. Neovascularization of some
advanced lesions is quite extensive, being derived from the adventitia. Thus, in the same subject one can see a wide spectrum of lesions. Some lesions are rich in
lipids and others are lipid-poor; some are macrophage-rich and others have only a rare macrophage, and smooth muscle cells are the dominant cell type. Still other
lesions are largely acellular. The picture may depend on the stage of lesion developmentor may even represent different mechanisms of lesion development. Some
atherosclerotic plaques contain genetic material similar to oncogenes that may induce a clonal growth of smooth muscle cells; in such lesions, smooth muscle cells
would predominate.
More than 90% of all myocardial infarctions are caused by the formation of a thrombus at the site of an atherosclerotic lesion. Most myocardial infarctions occur with
clot formation at sites that were narrowed less than that necessary to obstruct flow before the acute event (the average luminal narrowing in infarct arteries is about
50% to 55%). Thrombus formation usually occurs at sites of plaque rupture. Most plaques that rupture are in segments that contain arterial calcification, and the
rupture occurs in the shoulder region at the site of intense monocyte infiltration. Arterial calcification has been found to occur as a result of the induction of the same
set of genes as those induced in bone formation. Indeed, the calcification that results often cannot be distinguished from bone and may even include bone marrow.
Oxidized sterols and transforming growth factor b seem capable of inducing a subset of artery wall cells to undergo osteoblastic differentiation and form bone. As a
result of the presence of bone in the lesion, very high shear stress develops at the shoulders of the lesion at sites of inflammation, where monocyte-macrophages
release enzymes that destroy the normal arterial matrix. These areas rupture and expose the flowing blood to tissue factor that normally is expressed only in the
adventitia but is expressed in atherosclerotic lesions in the intima and media. The result is thrombus formation.
If the thrombus completely occludes the vessel lumen, an infarction occurs, unless collaterals are present to sustain viability. If the thrombus is only partially
occlusive, it may contribute to unstable angina, and it can later be incorporated into the plaque, causing further narrowing of the lumen. Unstable angina results from
the formation of platelet aggregates at these sites. The aggregates grow and obstruct flow, causing pain and electrocardiographic changes. In unstable angina,
however, the platelet plug is washed away before thrombosis is completed, thus averting a myocardial infarction.
ROLE OF LIPIDS AND LIPOPROTEINS
It is rare to find a patient with clinically important atherosclerosis without at least one of the following: a low-density lipoprotein (LDL) cholesterol concentration above
120 mg per dL; a high-density lipoprotein (HDL) cholesterol concentration below 40 mg per dL; triglyceride values above 175 mg per dL on fasting; and a
lipoprotein(a) [(Lp(a)] concentration above 30 mg per dL. Occasionally, patients have clinically important atherosclerosis without one of these findings, but almost
always, upon questioning, it is learned that the patient has changed his or her diet and lost weight before referral but shortly before or after the onset of symptoms.
The mechanisms by which these lipid abnormalities participate in the pathogenesis of atherosclerosis is an area of intense investigation.
Low-density lipoproteins are cholesteryl ester–rich lipoproteins that contain a large protein designated apolipoprotein B- 100 (apoB-100). This protein is synthesized
in the liver and has a molecular weight of about 500 kd. The gene for this protein has been isolated and sequenced. Portions of this protein mediate the binding of
LDL to heparin and to the LDL receptor. Predictably, abnormalities in this protein are being recognized as causes of hypercholesterolemia because of changes in the
receptor-binding domain. In most persons with atherosclerosis, LDL binds normally to normal LDL receptors. The concentration of apoB at sites of the arterial tree
where lesions are predictably found is substantially greater than the plasma concentration, indicating an accumulation of this protein at these sites. It appears that
arterial LDL accumulates at these sites because of an interaction between the extracellular components of the artery wall, such as proteoglycans and
glycosaminoglycans that bind LDL. Injection of LDL into a normal rabbit's femoral vein resulted in accumulation of the LDL in the subendothelial space within hours.
Ultrastructural studies demonstrated that the LDL was intimately associated with the matrix molecules that constitute the subendothelial space. This indicates that LDL
can cross an intact endothelium and bind to the extracellular matrix. The trapped LDL appears to be seeded with oxidative waste products from the artery wall cells.
As a consequence, an oxidized phospholipid is generated that induces the expression of the genes and proteins for an endothelial binding protein that binds
monocytes but not neutrophils or lymphocytes. This oxidized phospholipid also induces the artery wall cells to express the genes and proteins for monocyte
chemoattractant protein 1 (MCP-1) and macrophage colony-stimulating factor (M-CSF). As a result, monocytes bind to the endothelium, migrate down the MCP-1
gradient, and, under the influence of M-CSF, convert to macrophages. The macrophages releaseoxidative waste and oxidative enzymes that convert the mildly
oxidized LDL to highly oxidized LDL. The mildly oxidized LDL is recognized by the normal LDL receptor; the highly oxidized LDL is not but is recognized by scavenger
receptors and possibly an oxidized LDL receptor. In contrast to the LDL receptor, these latter receptors are not regulated by the cell's cholesterol content, and the
accumulation of cholesterol characteristic of foam cells occurs.
In contrast to LDLs, high-density lipoproteins (HDLs) have no apoB and are not substantially concentrated in the artery wall. The strong inverse relation between HDL
levels and atherosclerosis has been thought to be due to the role of HDL in reverse cholesterol transport. Although this hypothesis has many attractive features, it is
not proven, and the inverse relation of HDL cholesterol levels to atherosclerosis may be the result of unrelated factors. Some HDL particles (1% to 10%) carry
enzymes capable of destroying oxidized lipids; it may be that the content of these enzymes is critical to the protective effect of HDL. Another possibility relates to the
formation of HDL during the lipolysis of intestinally derived lipoproteins. High-density lipoproteins are formed as a by-product during the lipolysis of chylomicrons by
the enzyme lipoprotein lipase. Thus, in some subjects, low levels of HDL simply may reflect impaired lipolysis. Such subjects have low HDL cholesterol levels and
high triglyceride levels. The accelerated atherosclerosis frequently seen in such persons may not be due to a failure of reverse cholesterol transport as much as it is](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-111-320.jpg)



















![exercise training.
Chemoreceptors located in brain stem respiratory centers adjust respiratory amplitude and frequency on a moment-to-moment basis to ensure adequate oxygen
availability and carbon dioxide clearance in the blood. Longer-term changes in oxygen supply and demand are matched by finely tuned adjustments in the sensitivity
(i.e., gain) of chemoreceptors. With advancing age, chemosensitivity to oxygen and carbon dioxide tension declines, resulting in relative hypoventilation in response
to hypoxemia or hypercarbia. Therefore, older persons may be more vulnerable to vital organ ischemia during stresses such as surgery, acute pulmonary infections,
or high altitude, when oxygen availability is reduced.
ENERGY METABOLISM
Another critical physiologic function is the production of sufficient energy to meet the metabolic demands of the body. This process requires the intake and processing
of energy substrate (carbohydrate, fat, and protein) in the gastrointestinal tract; conversion of these substrates to simple sugars, fatty acids, or amino acids;
production of glucose or ketoacids by the liver for oxidative metabolism; insulin-mediated uptake of glucose by metabolically active cells; and participation in the
biochemical pathways leading to energy storage in high-energy phosphate bonds (i.e., adenosine triphosphate [ATP] production). Age-related changes in this
complex system have not been fully elucidated, and research has focused primarily on the summary measures of resting metabolic rate and daily energy expenditure.
Resting metabolic rate is usually determined by indirect calorimetry, which measures the rate of oxygen consumption (V O2) during quiet, supine rest under fasting and
thermoneutral conditions. The resting metabolic rate decreases with aging. Daily energy expenditure, which is measured by the doubly labeled water technique,
includes the resting metabolic rate, the thermic response to feeding, and the energy expenditure of physical activity. Daily energy expenditure also declines with
advancing age. However, the resting metabolic rate and daily energy expenditure are strongly influenced by physical fitness and activity, nutritional intake, and body
composition, all of which may change over time. Many of the changes in energy metabolism observed in the elderly may reflect altered physical activity and loss of
fat-free mass rather than biologic aging.
An exercise program that increases fat-free mass and energy intake can enhance energy expenditure in healthy elderly persons. Because the thermic effect of
feeding is higher in physically trained than inactive older men, much of the age-associated reduction in energy expenditure is probably attributable to the adoption of a
sedentary lifestyle, with its associated reduction in muscle mass.
Age-related changes in body composition may lead to a variety of disease states in the elderly. If energy intake remains constant despite reductions in physical
activity, older individuals accumulate body fat. After a 3-week period of overfeeding, young men develop hypophagia and lose their excess body weight, but older men
do not. This impairment in control of food intake and accumulation of body fat may lead to obesity, glucose intolerance, and hypertension.
The decline in glucose tolerance with advancing age has been well documented. It is manifested by modest elevations in fasting plasma glucose levels (approximately
1 mg per deciliter per decade) and marked elevations in 2-hour postprandial glucose levels (approximately 5 mg per liter per decade) during an oral glucose tolerance
test. The glucose intolerance of aging is related to peripheral insulin resistance, caused by a postreceptor defect in target tissue insulin action. There is no
age-related change in the number or affinity of insulin receptors or in maximal tissue responsiveness to insulin. However, healthy elderly subjects require larger
quantities of insulin to achieve a level of glucose uptake similar to that of the young ( Fig. 16.6).
FIGURE 16.6. Dose-response curves for insulin-mediated whole body glucose infusion rates in young ( dashed line) and old (solid line) subjects. A (left): Glucose
disposal is expressed as milligrams per kilogram of body weight. B (right): Glucose infusion rates are normalized for lean body mass. Elderly persons have reduced
sensitivity to insulin but no change in maximal glucose disposal. (From Rowe JW, Minaker KL, Pallotta JA. Characterization of the insulin resistance of aging.
Reproduced from J Clin Invest 1983;71:1581 by copyright permission of the American Society for Clinical Investigation.)
Studies of glucose-stimulated insulin secretion in healthy humans have shown impairments in insulin secretory capacity with advancing age. This is balanced by a
reduction in insulin clearance, the net result of which is no change in circulating insulin levels. However, the presence of “normal” circulating insulin levels in the face
of hyperglycemia suggests that insulin secretion is inappropriately low. Aging thus appears to be associated with insulin resistance and impaired insulin secretion.
Although insulin resistance was once thought to be a natural consequence of biologic aging independent of carbohydrate intake, body composition, or physical
activity, studies have shown elevations in body mass index and mean arterial blood pressure to be significant predictors of reduced insulin sensitivity, regardless of
age. Insulin resistance can be improved by exercise training. Glucose intolerance may also result from age-associated decreases in physical activity and fat-free
mass. Because of the association between chronic hyperglycemia and the development of atherosclerotic cardiovascular disease, renal disease, neuropathy, and
retinopathy, the glucose intolerance of advanced age has profound implications in the pathogenesis and prevention of disease in old age. This condition should not
be considered a harmless, age-related process; it should be treated as a significant risk factor for disability, which may be preventable through physical exercise and
proper nutrition.
DEFENSE SYSTEMS
The ability of the human body to defend itself from external pathogens and to prevent toxic effects of chemical exposures and metabolic by-products relies on the
presence of integrated physiologic networks that cross multiple organ systems. Many of the components of these networks are altered by the aging process, making
older persons more vulnerable to infectious disease, toxic drug effects, and malignancy ( Table 16.1).
TABLE 16.1. PHYSIOLOGIC DEFENSES AND THEIR CHANGES WITH AGE](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-131-320.jpg)
























![CHAPTER 22: PREVENTION OF NEOPLASIA
Kelley’s Textbook of Internal Medicine
CHAPTER 22: PREVENTION OF NEOPLASIA
PETER GREENWALD
Smoking and Cancer
Diet and Cancer
Lifestyle factors such as tobacco use and diet are major contributors to cancer risk. Because these lifestyle factors can be controlled, they are a logical focus for
cancer prevention strategies. Active participation in developing and carrying out such strategies by the medical community is critical to the success of cancer
prevention. The involvement of physicians in recognizing emerging issues (e.g., the increased prevalence of youth smoking) and encouraging patients to undertake a
healthier lifestyle (e.g., through adoption of a low-fat, high-fiber diet, decreased consumption of calories, and involvement in a regular exercise program) can result in
clear benefits to the individual patient as well as to society.
SMOKING AND CANCER
Developing effective cancer prevention strategies aimed at reducing tobacco use is an enormous public health challenge. Although the total prevalence of smoking
has declined in the United States over the past 30 years, tobacco use among adolescents is on the rise. Based on a 1995 survey, approximately 35% of all high
school students smoke—an increase of 27% from 1991 through 1995. In the black community, the prevalence of teenage smoking doubled between 1991 (14%) and
1995 (28%). Cigarette smoking is responsible for about one-third of all cancer deaths in the United States. Lung cancer, attributable primarily to cigarette smoking, is
the leading cause of cancer deaths among both men and women. Smoking also is a primary cause of cancers of the larynx, oral cavity, and esophagus and
contributes to cancers of the pancreas, bladder, kidney, uterine cervix, and renal system. Moreover, consistent evidence indicates that household or occupational
exposure of nonsmokers to environmental tobacco smoke (ETS) is associated with increased lung cancer risk. Although this increased risk is modest, the ubiquitous
nature of ETS translates into a large number of persons at risk for lung cancer or other respiratory illnesses.
Recent studies indicate that smokers with specific polymorphisms of the CYP1A1, CYP2D6, GSTM1, or MspI genes may have an increased risk of lung cancer
compared with those without these genetic variants. Also, particular alleles of the D2 dopamine receptor ( DRD2) may play a role in determining nicotine addiction and
may thus predispose persons to smoke.
TOBACCO DEPENDENCE
More than 70% of smokers would like to quit, and 60% have tried seriously to do so. However, addiction to nicotine—present in all tobacco products—makes quitting
difficult; 50% of smokers who have undergone major surgery for a tobacco-induced disease continue to smoke. Not all smokers exhibit the same degree of nicotine
dependence. Increased physical dependence is indicated by a high number of cigarettes smoked per day; frequent smoking in the morning; and smoking while ill.
Nicotine dependence must be given serious consideration when developing cancer prevention strategies related to tobacco use. Nicotine replacement therapies
(NRTs) should be considered, along with adjuvant behavioral counseling, to facilitate smoking cessation. In an effort to make NRTs more accessible, the nicotine
patch and nicotine gum were approved for over-the-counter (OTC) sale in 1996. The change from prescription to OTC use of NRTs resulted in more than twice as
many persons attempting smoking cessation in 1997 as in 1995. In addition, nicotine nasal spray, a nicotine inhaler, and buprion hydrochloride are available as
prescription medications to assist the smoker in quitting. All available pharmacotherapies appear equally effective; combining NRTs (e.g., patch and gum) may be
more effective than either method alone.
ROLE OF PHYSICIAN IN SMOKING PREVENTION AND CESSATION
Smoking prevention and cessation may represent the best means of reducing the total cancer burden. The importance of the physician's role in these efforts cannot
be overemphasized. At least 70% of smokers visit their primary health care provider at least once per year. A recent clinical practice guideline on smoking cessation
recommends that every patient who smokes be provided smoking cessation treatment at every office visit. Indeed, patients who smoke expect their physician to
inquire into their smoking habits and to advise them in cessation techniques. Studies indicate that 50% of all adult smokers started smoking by age 15, and 90%
started by age 18. Thus, early intervention, focused on preteens and teenagers, is critical to reducing the prevalence of adult smoking. Physicians who care for
children should anticipate the risk for tobacco use, ask about exposure to tobacco smoke and tobacco use, advise all smoking parents to stop smoking and all
children not to use tobacco products, and assist children in resisting tobacco use.
The National Cancer Institute (NCI) has formulated recommendations for physicians, outlined in Table 22.1, to help them institute smoking cessation techniques in
their medical practices. These recommendations are discussed in a step-by-step handbook— How to Help Your Patients Stop Smoking: A National Cancer Institute
Manual for Physicians—which includes resource lists, reprintable materials, and a special section, Clinical Intervention To Prevent Tobacco Use by Children and
Adolescents. The handbook is available on the internet at http://rex.nci.nih.gov/PREV__AND__ERLYDETC/PREVED__MAIN__DOC.html. Additional information on
smoking prevention, including items designed specifically for youth, is available at the Tobacco Information and Prevention Source (TIPS) Web page of the Centers
for Disease Control and Prevention (CDC) at http://www.cdc.gov/nccdphp/osh/.
TABLE 22.1. SYNOPSIS FOR PHYSICIANS: HOW TO HELP YOUR PATIENTS STOP SMOKING
DIET AND CANCER
A considerable body of evidence supports the hypothesis that dietary factors play a major role in the determination of cancer risk. Illustrating this point are the
significant international variations in the incidence of certain cancers as well as the increased risk observed when migrants from countries with a low-fat, high-fiber
diet adopt a more Western (high-fat, low-fiber) diet. For colon cancer, the Western diet is associated with increased disease risk in both men and women, especially
among those diagnosed before age 67 years. In contrast, consumption of the Mediterranean diet, which emphasizes vegetable, fruits, whole grains, fish, and olive oil
(high in monounsaturated fatty acids [MUFAs]), is thought to be cancer-protective, particularly for the digestive and respiratory tracts.
Data from analytic and experimental studies also support a relation between increased intakes of vegetables and fruits, fiber and whole grains, and certain
micronutrients and reduced cancer risk, whereas increased total caloric intake, body weight, and alcohol consumption are associated with greater risk. Overall risk of
developing cancer, however, cannot be separated readily into individual contributions from specific components of diet and diet-related factors, such as body weight
and exercise. All may interact to determine cancer risk. Individual genetic susceptibilities also may play an important role. To illustrate, among men who consumed
more than one serving of red meat per day, those with rapid acetylator polymorphisms ( NAT1, NAT2, or both) of the N-acetyltransferase gene were at increased risk](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-156-320.jpg)







![Several conditions for which screening cannot be justified in the general population should be sought when risk factors are identified as part of the baseline history or
physical examination. Such risk factors should be used to tailor the composition and frequency of the periodic health examination for individual patients. Such an
evaluation should include consideration of immunization status ( Chapter 26). Conditions that should be addressed in the periodic health examination of some patients
appear in Table 24.3. The list is not exhaustive. Inclusions and omissions may be controversial. In considering whether an intervention should be included in the
periodic health examination of a particular patient and the frequency of such examinations, the clinician should consider the criteria discussed, including those that
relate to characteristics and preferences of the patient.
TABLE 24.3. CONDITIONS THAT WARRANT PERIODIC EVALUATION OF SELECTED PATIENTS
BIBLIOGRAPHY
Canadian Task Force on the Periodic Health Examination. Canadian guide to clinical preventive healthcare. Ottawa: Canada Communication Group, 1994.
Coley CM, Barry MJ, Fleming C, et al. Early detection of prostate cancer: I, Prior probability and effectiveness of tests; II, Estimating the risks, benefits, and costs. Ann Intern Med
1997;126:394–406,468–479.
Eddy DM, ed. Common screening tests. Philadelphia: American College of Physicians, 1991.
Feinleb M, Zelen M. Some pitfalls in the evaluation of screening programs. Arch Environ Health 1969;19:412.
Hayward RSA, Steinberg EP, Ford DE, et al. Preventive care guidelines: 1991. Ann Intern Med 1991;114:758 [Erratum, Ann Intern Med 1991;115:332].
Kerlikowske K, Grady D, Rubin SM. Efficiency of screening mammography: a meta-analysis. JAMA 1995;273:149–154.
Mulley AG. Health maintenance and the role of screening. In: Goroll AH, May LA, Mulley AG, eds. Primary care medicine, third ed. Philadelphia: JB Lippincott, 1995.
Sox HC Jr. Preventive health services in adults. N Engl J Med 1994;330:1589.
US Preventive Services Task Force. Guide to clinical preventive services, second ed. Washington, DC: US Department of Health and Human Services, 1996.](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-164-320.jpg)









![CHAPTER 28: CANCER SCREENING AND EARLY DETECTION
Kelley’s Textbook of Internal Medicine
CHAPTER 28: CANCER SCREENING AND EARLY DETECTION
JHON K. GOHAGAN, BARNETT S. KRAMER AND WILLIAM C. BLACK
Principles of Cancer Screening
Screening for Specific Cancers
Assessment of Screening Effectiveness
PRINCIPLES OF CANCER SCREENING
Several methods of cancer screening have proved effective in decreasing cancer mortality. Dramatic technological advances in imaging and molecular detection
during the last two decades have greatly enhanced the opportunities for cancer screening in the future. However, there is much confusion about assessing
effectiveness of early detection and the net benefit of specific screening strategies. Experience has shown that arguments for earlier cancer detection based on
advances in detection technologies, disease risk, or treatment advances should be treated as plausible hypotheses to be tested with scientific rigor.
Early detection of cancer does not axiomatically confer benefit and may confer harm. For early detection by screening to be appropriate, two consequent conditions
must be met:
Treatment provided at the time of screen detection must be more effective in reducing cause-specific mortality than treatment provided at the usual time of
clinical diagnosis.
The benefits of earlier treatment must outweigh the harms derivative to screening.
The first condition is very difficult to determine. Survival measured in screen detected versus clinically detected cases overestimates effectiveness. The second
condition is also very difficult to determine, and the potential for harm is often not recognized by screening advocates. False-positive screening tests often lead to
invasive procedures to exclude malignancy. When early detection does not change the course of the disease, the patient suffers the effects of diagnosis and
treatment for a longer period of time. This latter harm is particularly relevant to the detection of premalignant lesions and carcinoma in situ, many of which might never
have become clinically significant.
Some organizations apply categorical quality levels to sources of evidence regarding screening effectiveness. The National Cancer Institute's Physician Data Query
system uses a five-level categorization, in declining order of strength, similar to the one listed below. The US Preventive Services Task Force, the Canadian Task
Force on the Periodic Health Examination, and the American College of Physicians use similar rating systems.
Randomized controlled trials
Internally controlled trials without randomization (e.g., allocation by birth date)
Cohort or case-control studies
Multiple-time series with or without intervention
Opinions of respected authorities based on clinical experience, descriptive studies, or reports of expert committees
Randomized controlled trials that correct for or eliminate several confounding biases provide the most compelling scientific evidence.
Selection bias can cause either under- or overestimation of screening effectiveness, depending on whether those at higher risk of the disease are more or less likely
to undergo screening. Lead-time bias pertains to comparisons that are not adjusted for the timing of diagnosis. If screen-detected cases are diagnosed earlier, they
will survive longer from the time of diagnosis even if their death is not delayed. Length bias pertains to comparisons that are not adjusted for the rate of disease
progression. Cases detected by screening are more likely to be slowly progressive than those not detected by screening and ultimately presented clinically.
Overdiagnosis bias pertains to comparisons that are not adjusted for the detection of pseudodisease, preclinical disease that would not have produced any signs or
symptoms before the person would have died from other causes. Overdiagnosis bias, which can be considered an extreme form of length bias, can markedly inflate
the survival and cure rates in screen-detected cases ( Fig. 28.1, Fig. 28.2 and Fig. 28.3).
FIGURE 28.1. Selection bias results from the tendency of more health-conscious persons with mortality rates lower than expected to join screening programs. (From
Kramer BK, et al. Prostate cancer screening. Ann Intern Med 1993;119:917, with permission.)
FIGURE 28.2. Lead time bias. Without screening, diagnosis occurs when clinical signs or symptoms develop. With screening, time of diagnosis is advanced by lead
time provided by positive test result. If earlier diagnosis has no effect on time of death from disease, then survival with testing is equal to survival without testing plus
lead time. (From Black WC, Welch HG. Screening for disease. Am J Roentgenol 1997;168[1]:3–11;www.arrs.org, with permission.)](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-174-320.jpg)
![FIGURE 28.3. Length bias. Probability of detection is related to rate of disease progression. The length of each arrow represents length of detectable preclinical
phase, from initial detectability to clinical diagnosis ( Dx). Testing at a single moment in time detects four slowly progressive cases, but only two rapidly progressive
cases (bold arrows). Cases not detected by test (gray arrows) are diagnosed clinically, either before or after time of testing. (From Black WC, Welch HG. Screening
for disease. Am J Roentgenol 1997;168[1]:3–11;www.arrs.org, with permission.)
SCREENING FOR SPECIFIC CANCERS
BREAST CANCER
In 1999, an estimated 176,300 new cases of breast cancer will be detected in the United States. Incidence increased at an annual rate of 1% between 1940 and 1980
and has been increasing at a substantially higher rate since 1980 with the widespread application of screening mammography. Recent data suggest that incidence
may be leveling off. In 1999, an estimated 43,700 breast cancer deaths are projected.
Data from the world's eight randomized breast cancer screening trials were reviewed and critiqued at the National Institutes of Health (NIH) Consenus Development
Conference of January 21–23, 1997. The NIH Consensus Statement on Breast Cancer Screening for Women Ages 40–49 contained two reports. The Majority Report
stated, “The Panel concludes that the data currently available do not warrant a universal recommendation for mammography for all women in their forties.” Two panel
members wrote a Minority Report in which they expressed an opinion that the risks of mammography were overstated by the majority and that current data supported
screening mammography for all women ages 40 to 49.
One major concern of the NIH Consensus Panel was the detection of ductal carcinoma in situ (DCIS), which may progress to invasive cancer very slowly or not
progress at all. DCIS constitutes about 50% of all mammographically detected malignancies in women 40 to 49 years of age, and the age-adjusted incidence of DCIS
has increased more than threefold since 1983, along with the increased use of mammography.
Controversy continues regarding the appropriateness of periodic mammographic screening of women ages 40 to 49 years.
The Consensus Panel's report did not address the issues of screening mammography for women aged 50 and older. Previously, the National Cancer Institute's
International Workshop on Screening for Breast Cancer (February 24–25, 1993) concluded that there is strong evidence from clinical trials that, “between 50 and 69
years of age, screening with mammography alone or in combination with clinical breast examination at 12- to 33-month intervals can reduce mortality by about 30%.”
The effectiveness of screening beyond 70 years of age is unknown.
PROSTATE CANCER
An estimated 179,300 new cases of prostate cancer and 37,000 prostate cancer-related deaths are projected for 1999 in the United States. It is projected that prostate
cancer will account for 29% of all male cancers and 13% of male cancer deaths in the United States in 1999.
The rate of progression of prostate cancer is highly variable and unpredictable. Clinical prostate cancer is rare in men younger than 50 years of age, although occult
cancer is common in autopsy series of men who died of causes unrelated to prostate cancer. Incidence rises rapidly after 50 years of age. Age-adjusted incidence
among blacks (142.0 cases per 100,000 men) is substantially higher than among whites (108.3 cases per 100,000 men). Men with a family history of prostate cancer
are thought to have an increased risk of the disease compared with men without this history.
Widespread prostate cancer screening in the United States has led to dramatic increases in prostate cancers detected in the United States compared with the United
Kingdom where screening is less intense. However, prostate cancer mortality in the two countries is nearly identical.
Predictions of the effect of prostate cancer screening from mathematical models range from net benefit to net harm. Opinions based on clinical experience, descriptive
studies, or reports of expert committees therefore vary from endorsement to specific recommendations against population screening. Randomized trials designed to
ascertain the risk-to-benefit balance will not report results for several years.
COLORECTAL CANCER
Colorectal cancer is the second leading cause of death from cancer in the United States. Approximately 129,400 new cases and 56,600 deaths in the United States
are projected for 1999. Incidence is higher among men than women. Both incidence and mortality rates in the United States are declining for reasons that may include
dietary changes, improved treatment, and screening.
Screening for colorectal cancer represents a form of primary and secondary prevention, because it can lead to the identification and eradication of preneoplastic
lesions such as adenomatous polyps.
Hereditary risk factors, such as familial polyposis, hereditary nonpolyposis syndrome, the cancer family syndrome (autosomal dominant), and hereditary site-specific
colon cancer together may account for less than 10% of colorectal cancers. Other risk factors include a personal or first-degree family history of colorectal cancer or
adenomas, inflammatory bowel disease (especially ulcerative colitis), and a personal history of ovarian, endometrial, or breast cancer. High-risk groups may account
for as much as 23% of all colorectal cancers.
A large percentage of early cancers and precursor adenomas can be detected by screening asymptomatic persons older than 50 years of age using the fecal occult
blood test and flexible sigmoidoscopy. It is hypothesized that removal of premalignant adenomatous polyps should decrease mortality, although direct evidence from a
randomized clinical trial is not available. The discovery of polyps in the distal colon or rectum or the presence of occult blood mandates colonoscopy to search the
entire colon.
Two case-control studies evaluated the association of screening sigmoidoscopy with a decrease in colorectal cancer mortality. One study evaluated rigid
sigmoidoscopy; the other evaluated rigid and flexible sigmoidoscopy. Both studies suggested a significantly decreased risk (60% to 80%) of fatal cancer of the distal
colon or rectum among persons with a history of one or more sigmoidoscopic examinations compared with nonscreened patients. The decrease in colorectal cancer
associated with screening was restricted to cancers that occurred within the reach of a sigmoidoscope.
Several controlled clinical trials are evaluating the efficacy of screening using the fecal occult blood test (FOBT). The Minnesota trial demonstrated that annual FOBT
using rehydrated samples decreased mortality from colorectal cancer by 33%; biennial FOBT yielded a 21% reduction. Randomized trials in Denmark and the United
Kingdom also report mortality reductions with biennial FOBT screening.](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-175-320.jpg)




![as that for patients with established CVD (less than 100 mg per deciliter). Niacin is an effective therapy for patients with low HDL cholesterol and elevated
triglycerides, a common pattern in diabetics; however, because niacin may be associated with a worsening of glucose tolerance, statins are often the preferred
therapy. Proper nutrition, weight management, and regular exercise are the mainstay of treatment for diabetics, as is control of concomitant risk factors.
Obesity
The number of men and women who are obese is substantial and on the rise. Nearly 60% of white men and 45% of white women aged 20 to 74 years are overweight
[body mass index (BMI) between 25 and 30 kg per square meter], and 20% and 22.4%, respectively, are obese (BMI ³30 kg per square meter). The prevalence of
obesity is greater among blacks and is as high as 67% among Mexican Americans. There is a linear relationship between BMI and CHD. Central obesity
(intra-abdominal fat) is a stronger predictor than peripherally deposited fat. Measurement of waist circumference is useful as a diagnostic aide. A substantial amount
of the risk associated with obesity is mediated through high blood pressure, dyslipidemia, diabetes, and insulin resistance. Gradual (1 to 2 pounds per week) and
sustained weight loss in persons whose weight exceeds the ideal for their height should be encouraged through caloric restriction and increased physical activity.
Sedentary Lifestyle
A sedentary lifestyle is associated with a doubling of CHD risk compared with a more active lifestyle, even when other factors are taken into consideration. About 25%
of adults report no regular leisure-time activity. Physical inactivity is more common among women than men, greater among older than younger adults, and more
prevalent among ethnic minorities and the less educated and less affluent. Physical inactivity increases the risk of high blood pressure 30% to 50%. In contrast,
regular physical activity is associated with reduced cholesterol and increased HDL levels, lower triglycerides, enhanced fibrinolysis, improved insulin sensitivity, and a
lower incidence of diabetes. Moreover, exercise may slow progression of atherosclerosis, improve myocardial oxygen delivery, enhance myocardial function, and
reduce vulnerability to dysrhythmias.
The greatest differential in CHD risk appears to be between those who are completely sedentary and those who exercise moderately on a regular basis. Recent
evidence suggests that regular activity of moderate intensity, including brisk walking, is associated with a significant reduction in CHD risk. These data support the
1995 federal exercise guidelines endorsing 30 minutes of moderately intense physical activity most days of the week, a safe and feasible strategy for most of the
population. For patients who have experienced cardiovascular events or undergone revascularization procedures, participation in formal cardiac rehabilitation or a
physician-guided home exercise program should be encouraged.
Dietary Factors
Diet is a major cause of CHD. Dietary intake of fat, especially saturated fat, and cholesterol is strongly correlated with cholesterol concentrations and CHD risk.
Saturated fat intake is also associated with increased thrombogenicity. Intake of trans fatty acids has been linked to adverse lipid profiles and increased risk of CHD.
Salt and alcohol intake influences blood pressure. Moderate alcohol intake (one to two glasses per day) is associated with increased HDL and reduced CHD risk, but
it is not generally recommended as a prevention strategy because of the potential for abuse and increased CVD risk at higher intakes. Increased ingestion of fiber,
whole grains, and fruits and vegetables is cardio- protective. Diets rich in antioxidant nutrients, such as vitamin E, asorbic acid, and beta carotene, have been
associated with a reduced risk of CHD in epidemiologic studies; however, clinical trials of antioxidant supplementation have been inconsistent. The American Heart
Association has recommended that total fat intake not exceed 30%, that saturated fat intake be 8% to 10% of calories, and that cholesterol intake be less than 300 mg
per day. Further reductions in saturated fat and cholesterol are indicated in those with CVD or lipid disorders ( Table 29.3 and Table 29.4). Total dietary fiber intake
should be 25 to 30 g per day, salt intake should be limited to 6 g per day, alcohol should be limited to two glasses per day (one glass for women), and five or more
servings of fruits and vegetables should be consumed daily.
Pyschosocial Factors
Several psychosocial factors are associated with CHD risk, but there is a paucity of data to verify that improvements in psychosocial parameters lower risk. The type A
personality is a good example, with hostility traits also emerging as a consistent factor. Social isolation and features that contribute to it, such as low education level,
adverse economic circumstances, and lack of family and social networks, are associated with increased CHD risk. Depression, especially following MI, increases
coronary risk. Chronic stress, particularly in situations of “high demand and low control,” may double the risk of MI. Acute psychological stress may also trigger MI.
Psychosocial risk factors are common and their assessment should be part of a routine screening evaluation for CHD. Intervention may improve quality of life and
adherence to medical regimens and potentially lower mortality rates and recurrent CHD events.
New Risk Factors
Well over 200 risk factors have been described for CHD. Only a small fraction of them are classified as major risk factors, because they are not considered
independent and causal, because they are modifiable or have a low prevalence, or because there is no reliable screening test or effective intervention. Recently,
levels of small, dense LDL particles, insulin, lipoprotein(a), C-reactive protein, Chlamydia pneumoniae antibody titers, homocysteine, dehydroepiandrosterone sulfate,
oxidative stress, fibrinogen, plasma viscosity, and a number of other thrombotic factors (plasminogen activator inhibitor type 1, tissue-type plasminogen activator
antigen, von Willebrand's factor, factor VIIa) have been correlated with CHD risk. The current role of screening for these factors in practice is limited, owing to the
reasons cited earlier. Moreover, the added value of measurement of new risk factors over traditional risk factors in identifying individuals at risk of CHD or in
developing risk-reduction strategies is not established, but it is unlikely to be substantial. In certain clinical situations or in the case of unexplained or premature CHD,
however, selective determination of novel risk factors may be useful to physicians and patients.
COMPREHENSIVE PREVENTIVE CARDIOLOGY
A shift in paradigms from a reactive approach to CVD and its side effects to prescribing prevention is necessary to reduce the burden of the disease. Several
strategies for prevention are available to guide the physician in determining the type and aggressiveness of interventions in various patients. In healthy patients
without known risk factors for CVD, an emphasis should be placed on developing and maintaining healthy lifestyle habits in an effort to prevent the evolution of such
risk factors as high blood pressure and hypercholesterolemia. Primary prevention is the term applied to prevention among individuals free of clinical CVD who are
susceptible to it because they have a risk factor. Lifestyle modification is also emphasized in this context, as is appropriate treatment of risk factors, especially among
individuals with multiple risk factors ( Table 29.3). Patients with established CVD (secondary prevention) are at greatest risk of a future event and therefore should be
treated most aggressively (Table 29.4).
The risk/benefit ratio of aggressive risk factor management is most favorable among those with the highest level of baseline risk. An overlapping area of risk occurs in
patients without clinical manifestations of CVD but with evidence of significant disease based on a screening procedure such as carotid ultrasound or ultrafast
computed tomography scanning of the coronary arteries. Because the number of noninvasive tests to identify patients with CVD is increasing and because the
predictive value of these tests is often not firmly established, interpretation of the results can present a challenge. Aggressive risk factor management has been shown
to prevent the progression of atherosclerosis; therefore, it may be prudent to treat subclinical disease more aggressively than would occur in the context of primary
prevention. Because one-third of coronary events are fatal, it is also important to prioritize risk factor management before an event occurs.
There is abundant evidence of missed opportunities to institute the consensus recommendations for the prevention of CVD outlined in Table 29.3 and Table 29.4.
National data have shown that only a small proportion of patients receive counseling about lifestyle risk factors in ambulatory care settings. As described previously,
there are adverse trends in several major risk factors for CHD, and many patients are not being treated to target risk factor levels. Only a small fraction of patients who
qualify for cardiac rehabilitation are referred, despite a 25% improvement in survival rates associated with participation. Cardiac rehabilitation is a key step in initiating
risk factor management and restoring functional capacity after an MI or revascularization procedure. It is a model for a comprehensive prevention program, yet the
reasons for its underutilization are not fully understood. Barriers to the spectrum of prevention strategies may be related to patients, physicians, the lack of
organizational structure, or societal factors. Communication, training, infrastructure, and reimbursement for preventive services will undoubtedly help patients achieve
risk factor targets that have been shown to reduce the burden of CVD. A multidisciplinary team approach that includes nonphysician health care providers may be an
especially cost-effective approach to the prevention of CVD.
BIBLIOGRAPHY
Grundy SM, Balady GJ, Criqui MH, et al. Guide to primary prevention of cardiovascular diseases: a statement for healthcare professionals from the Task Force on Risk Reduction. Circulation](https://image.slidesharecdn.com/nternal-medicinetextbook-220720191359-9646fce3/85/nternal-medicine-textbook-pdf-180-320.jpg)