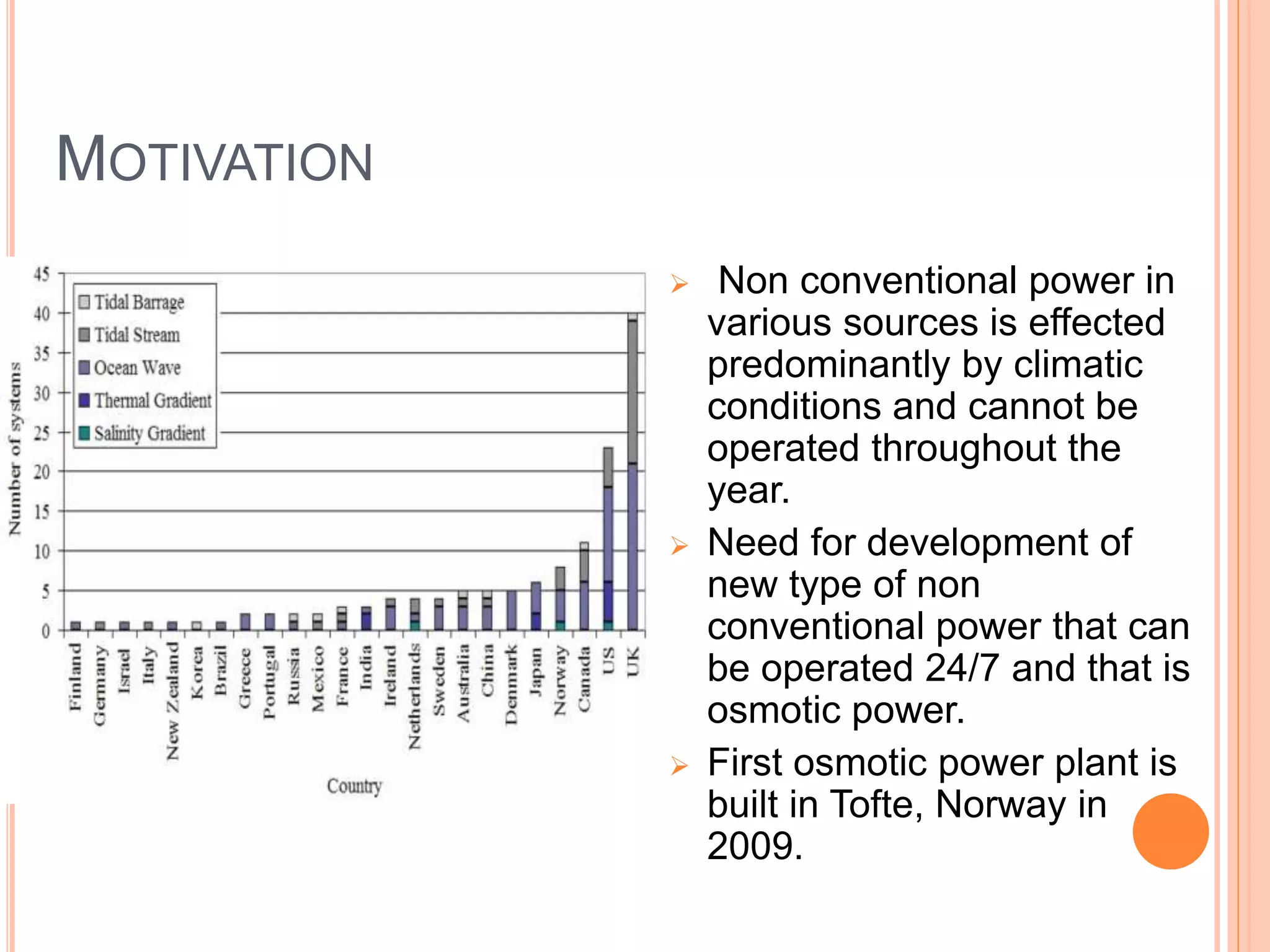
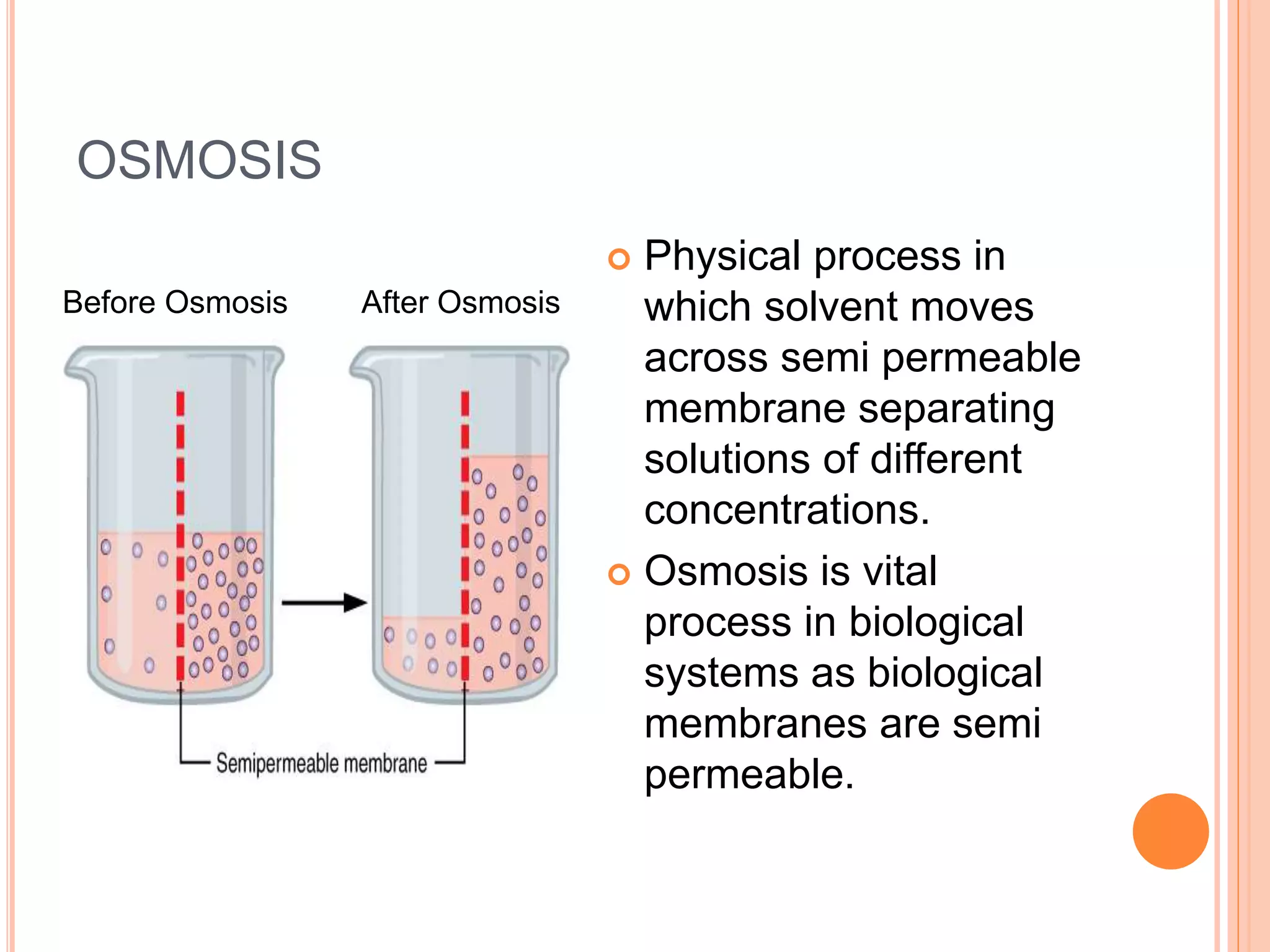
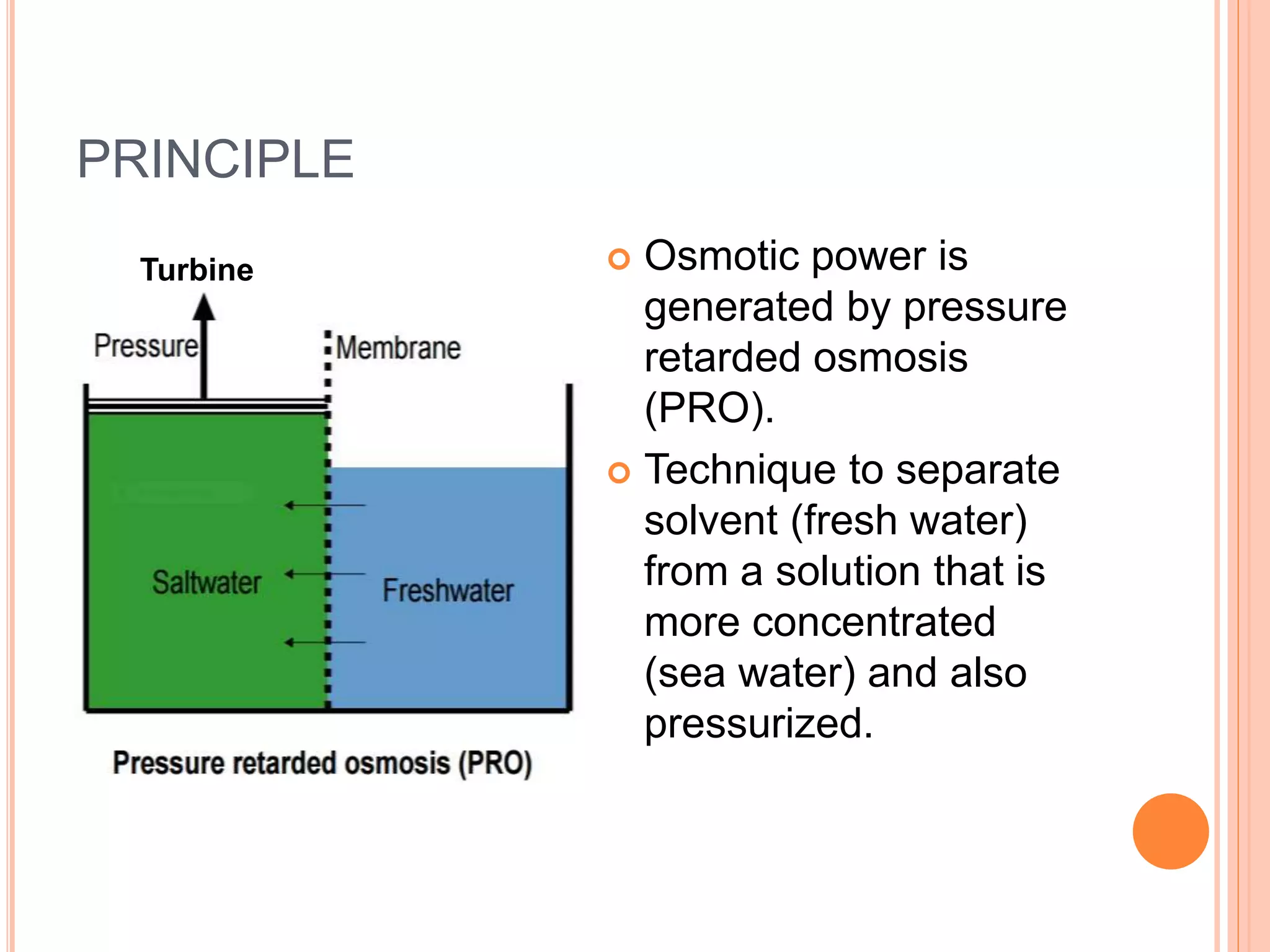
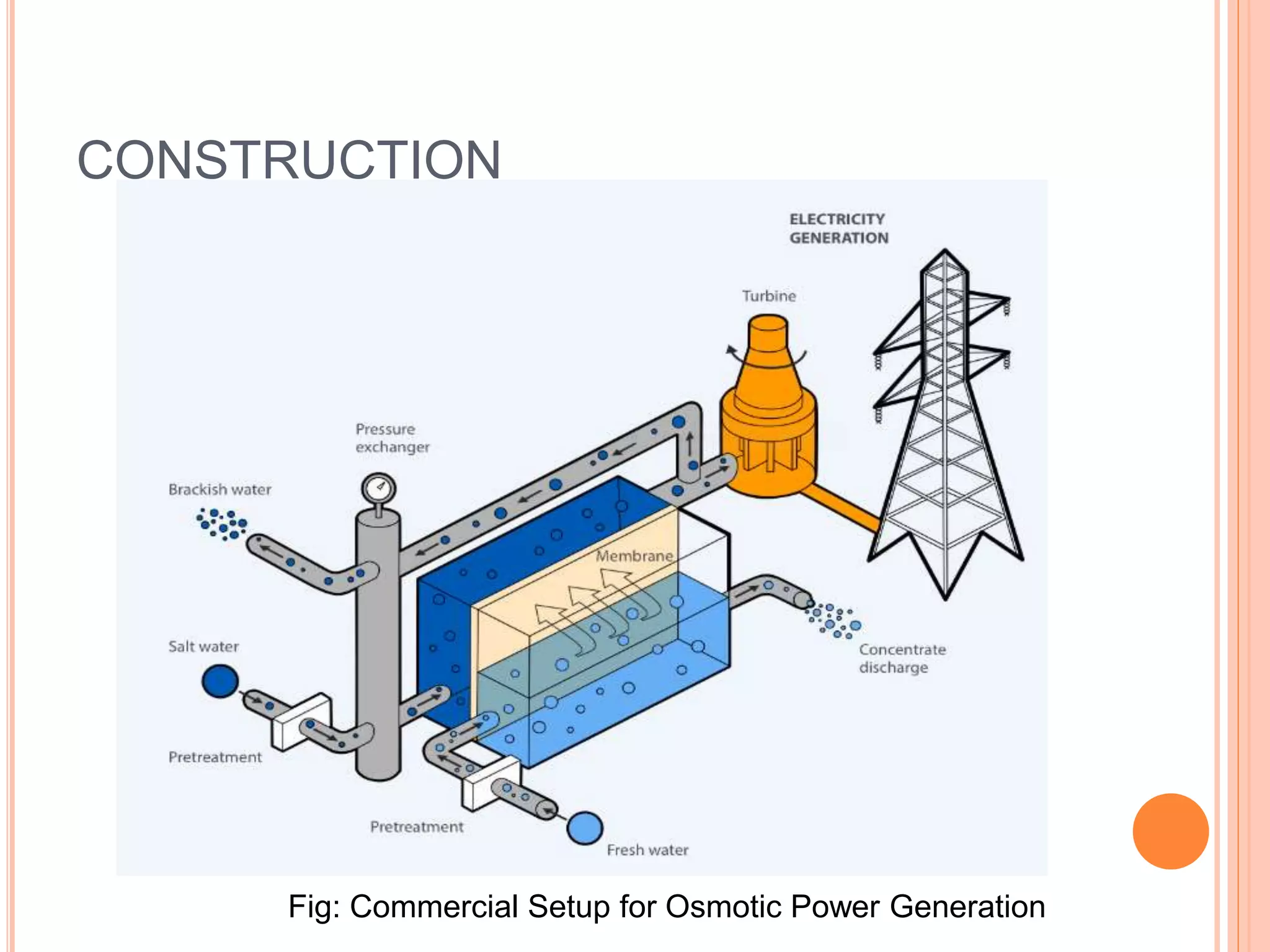
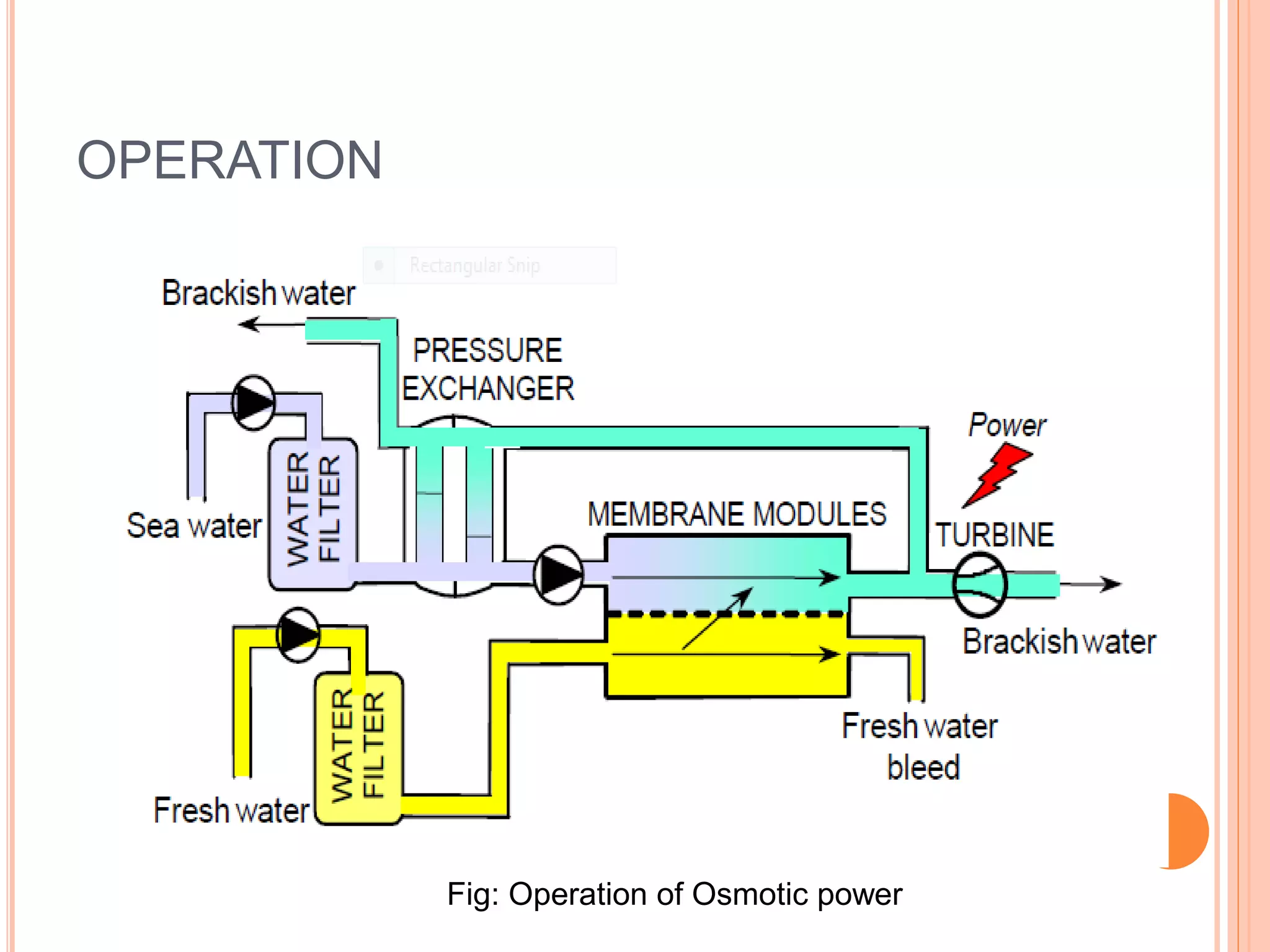
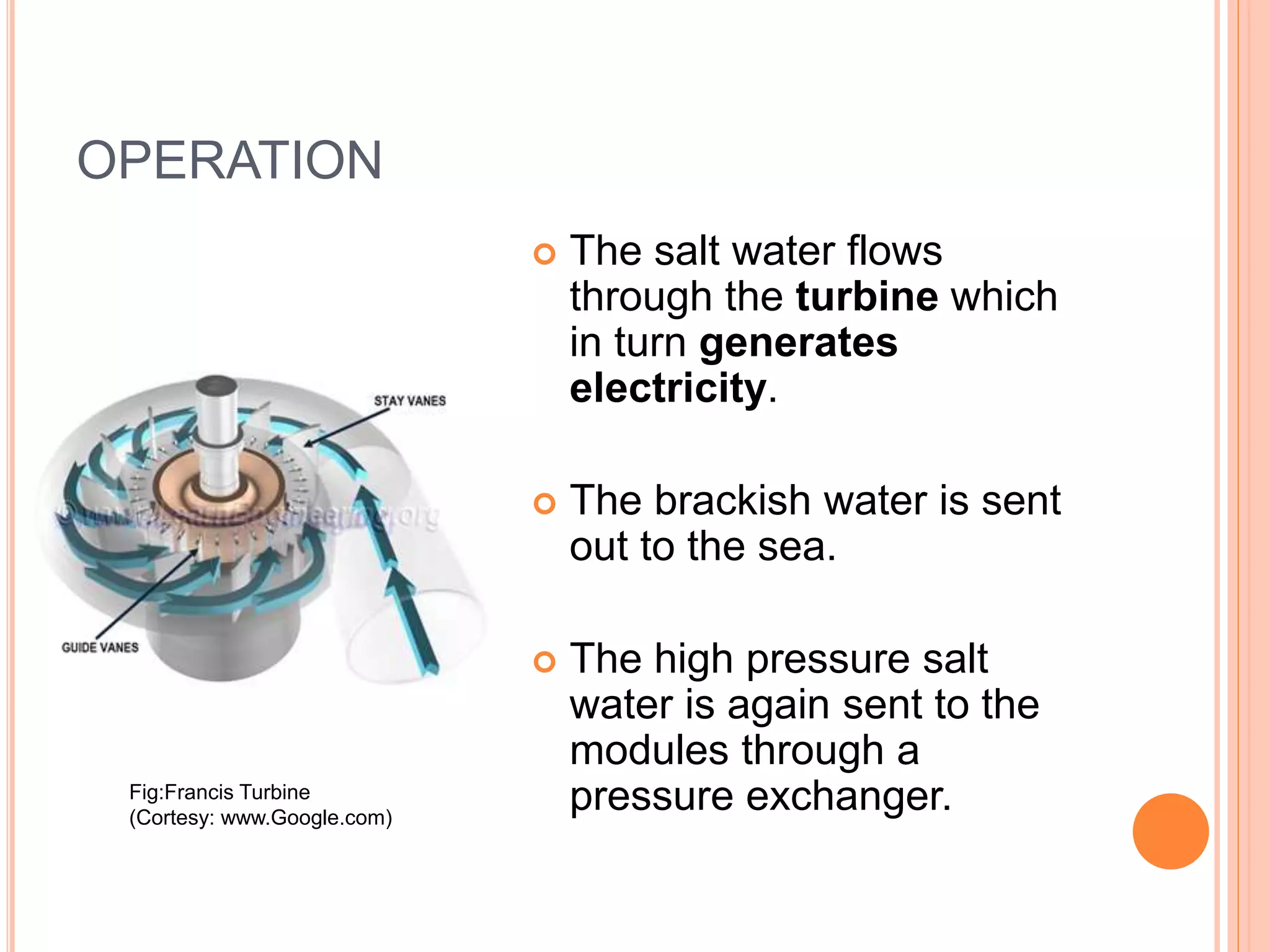
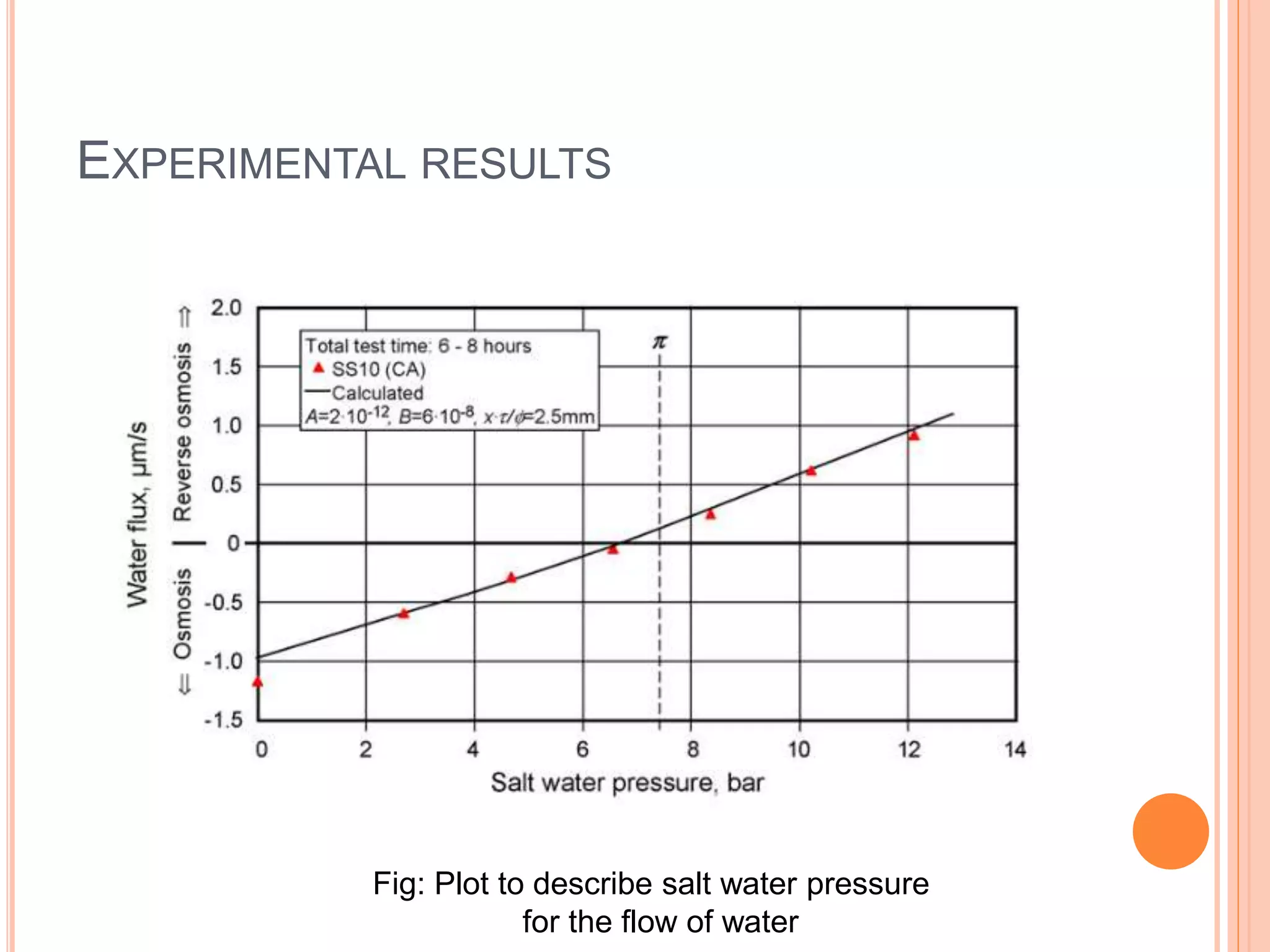
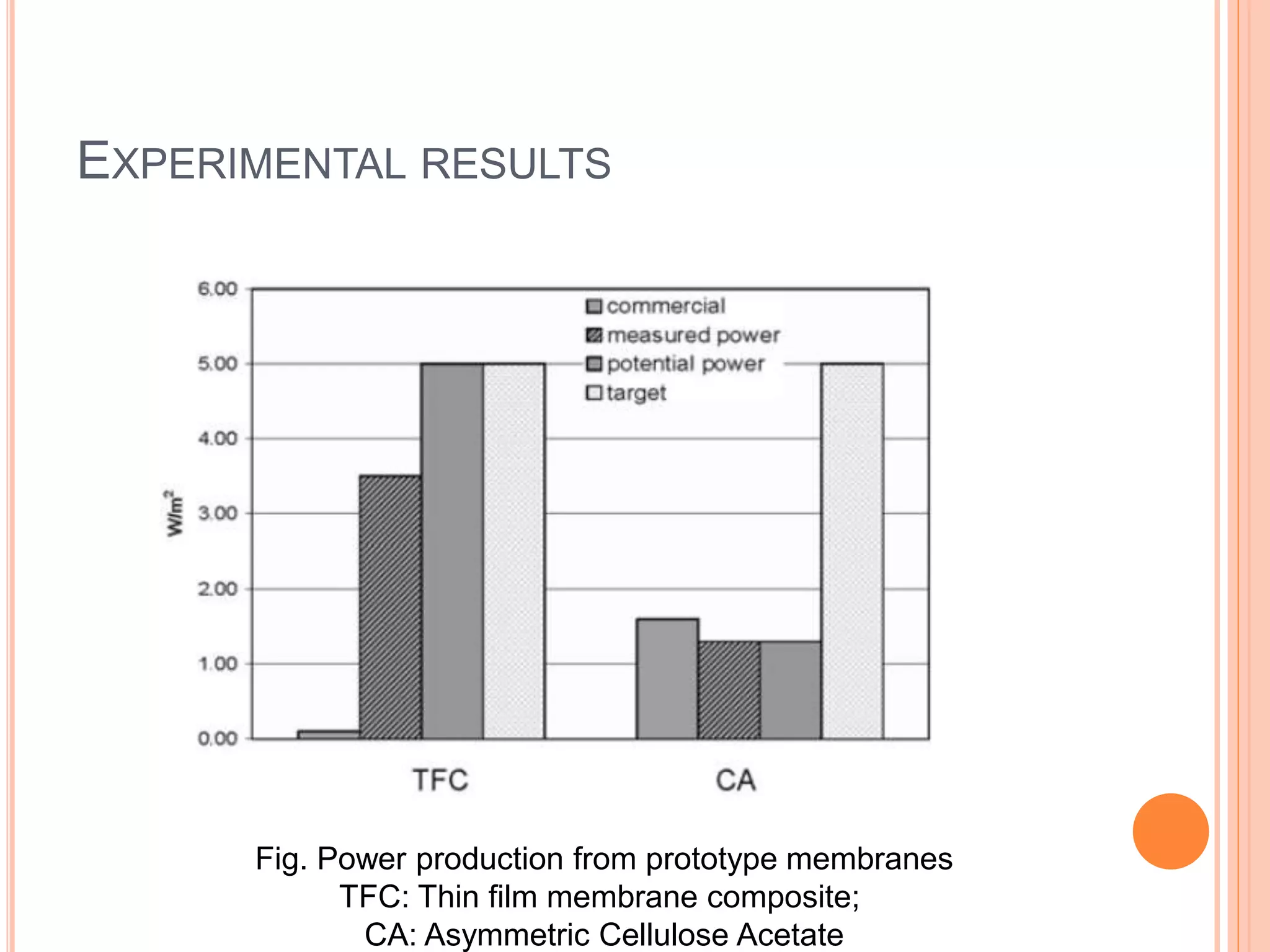
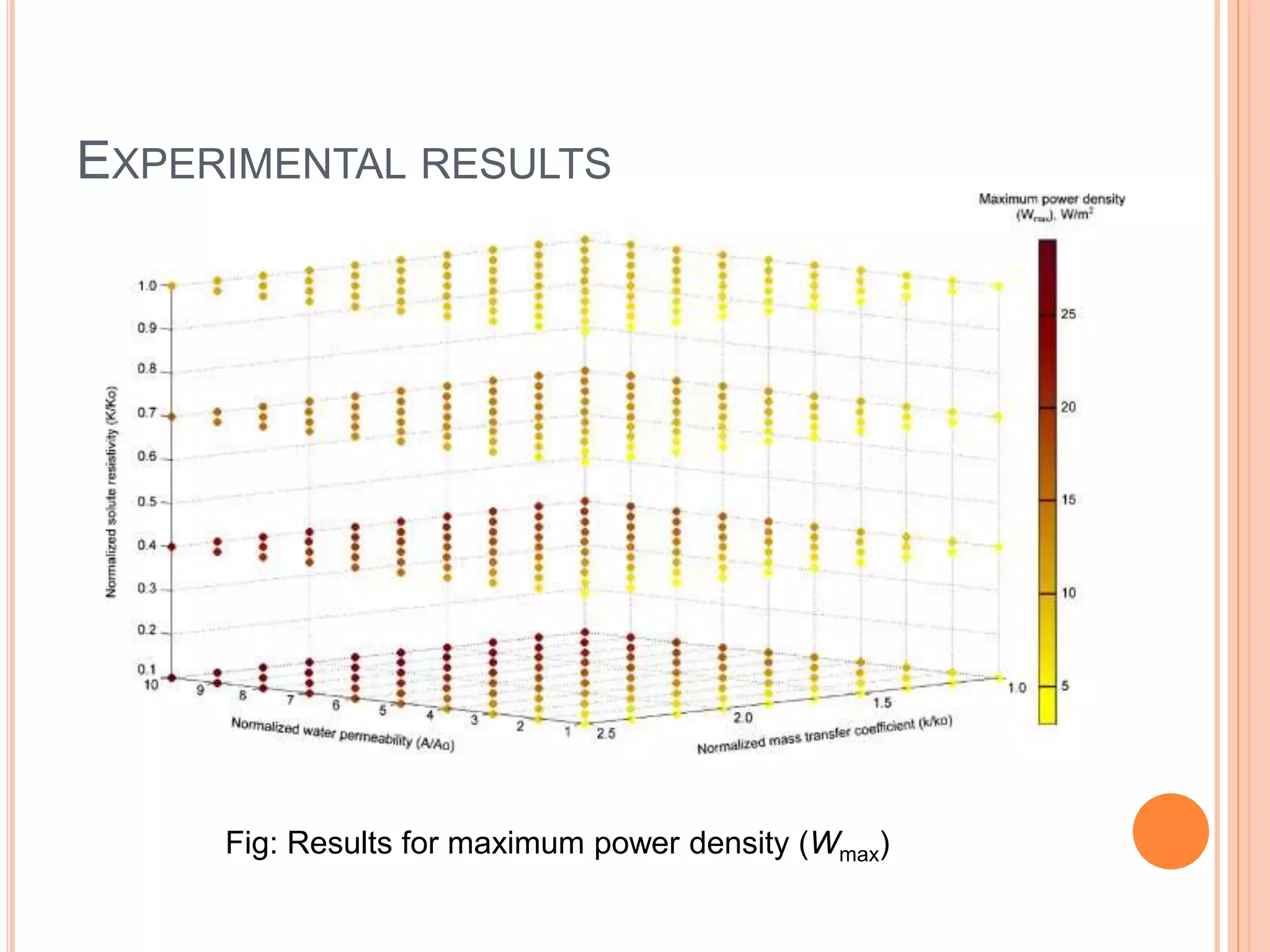
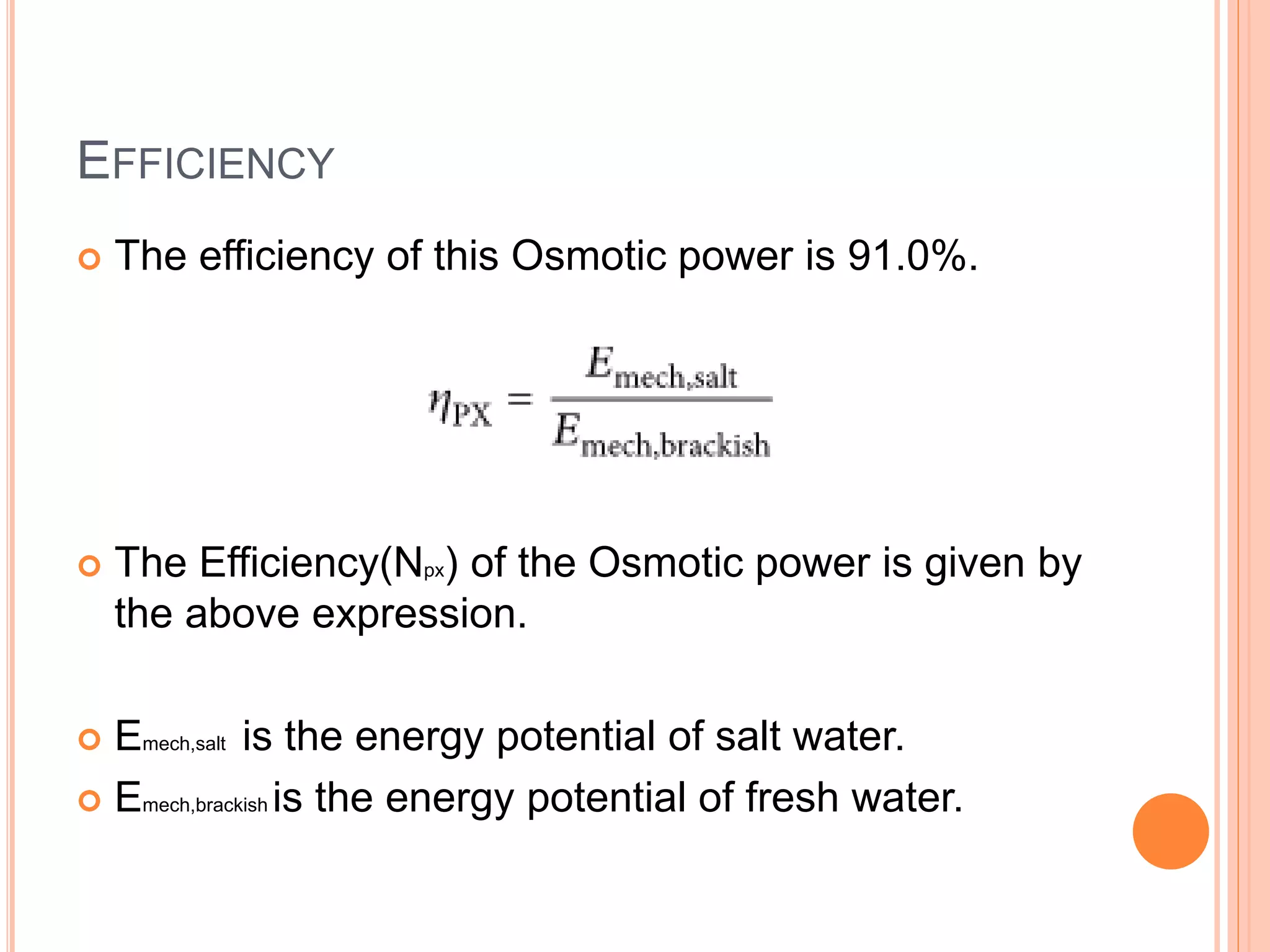
This document outlines osmotic power, which generates energy from the difference in salt concentration between seawater and freshwater. It works via pressure retarded osmosis (PRO) where freshwater naturally moves through a semi-permeable membrane into higher salinity seawater, increasing pressure. This pressure powers a turbine to generate electricity. Key components include membrane modules to separate the waters, filters to optimize membrane performance, and a turbine/generator. Experimental results showed a prototype achieving over 90% efficiency and the potential to scale installations by adding more membrane modules.