Embed presentation
Downloaded 494 times









The presentation discusses strategies to optimize preclinical proof of concept to improve the success of drug development, which has a low overall success rate of less than 10%. It emphasizes the importance of developing a comprehensive plan, utilizing relevant animal models, understanding the targeted disease physiology, and incorporating feedback for effective commercialization. Critical factors for early development include selecting appropriate indications, assessing toxicity, and ensuring data quality to support regulatory filings and future clinical trials.
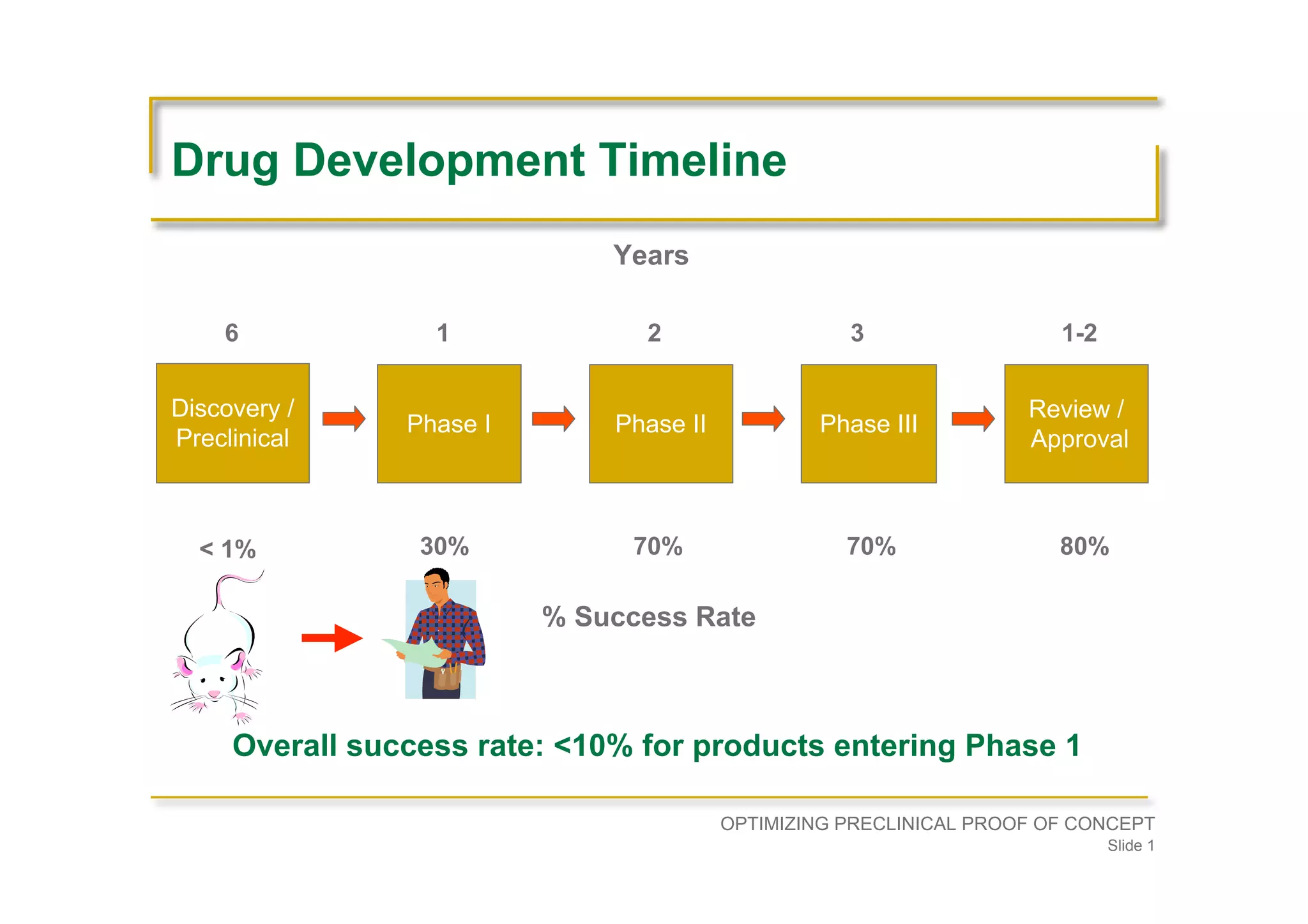
Overview of drug development stages, timelines, and the low overall success rate (<10%) for products entering clinical trials.
Identifies key reasons for drug failures: toxicity (49%), unpredictability in bioavailability (15%), metabolism issues (3%), and faulty understanding of pathophysiology (33%).
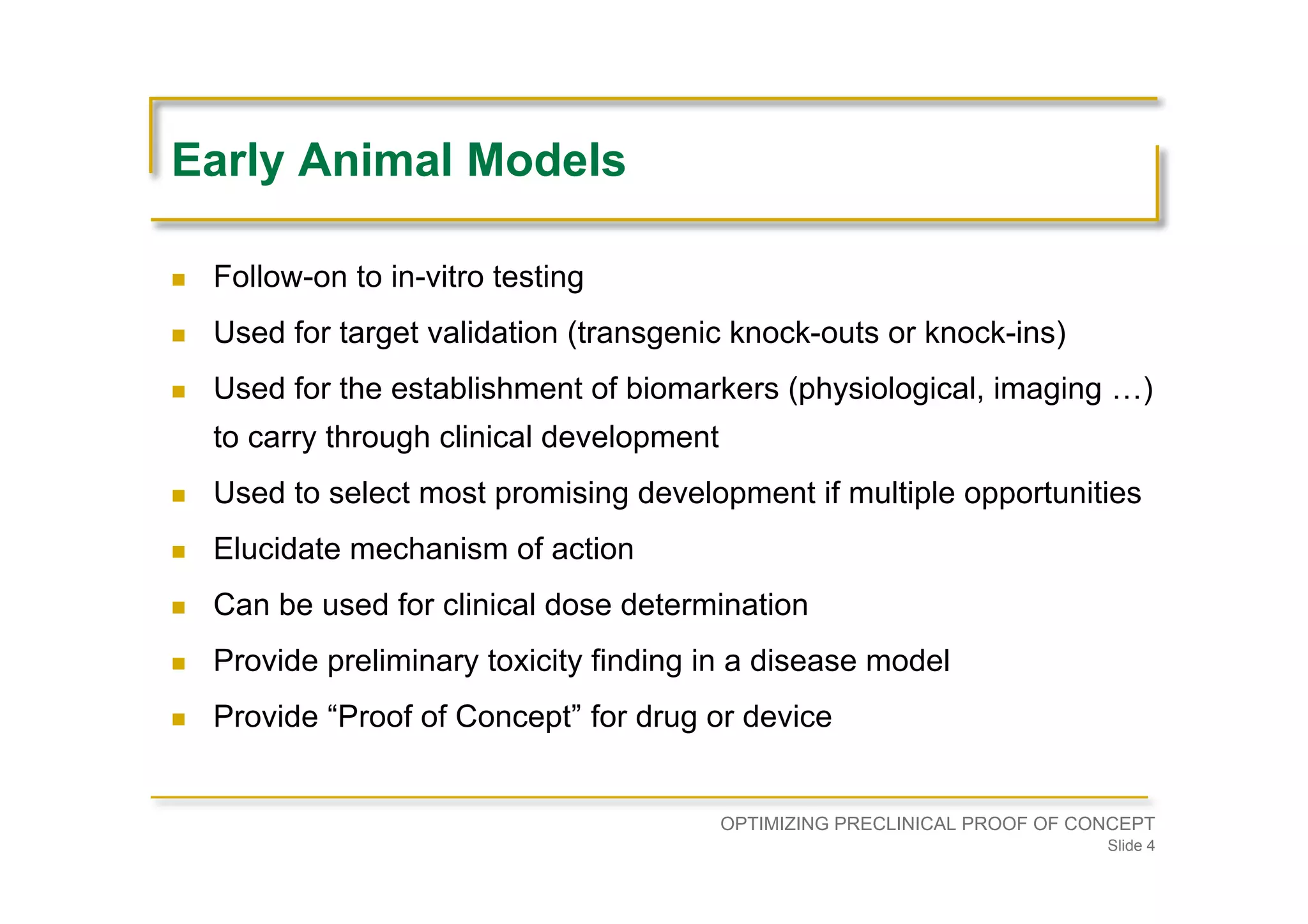
Discusses the importance of early animal models for target validation, biomarker establishment, selecting promising developments, and elucidating mechanisms of action.
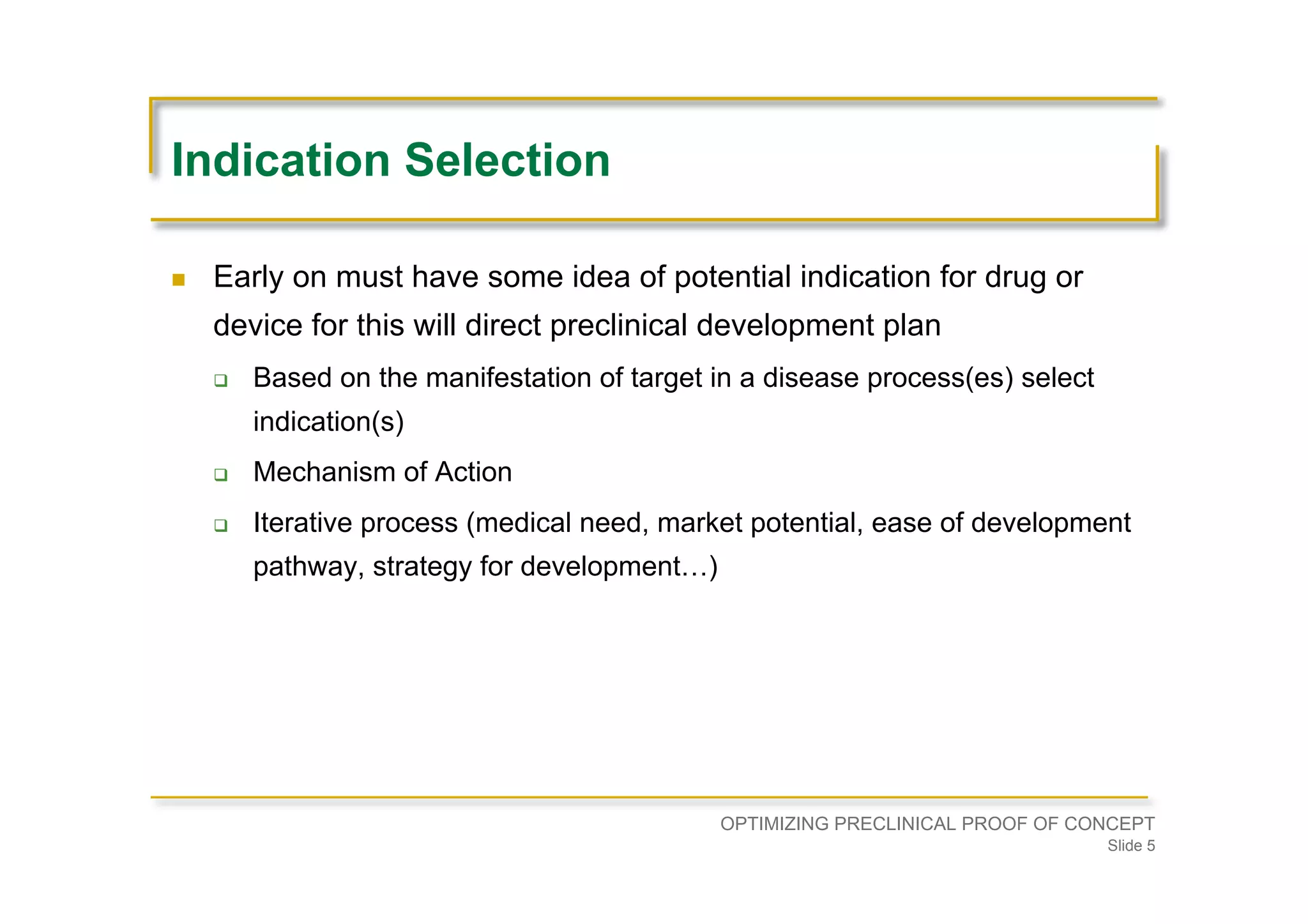
Highlights the need for potential indication identification early in development, based on target manifestation, mechanism of action, medical need, and market potential.
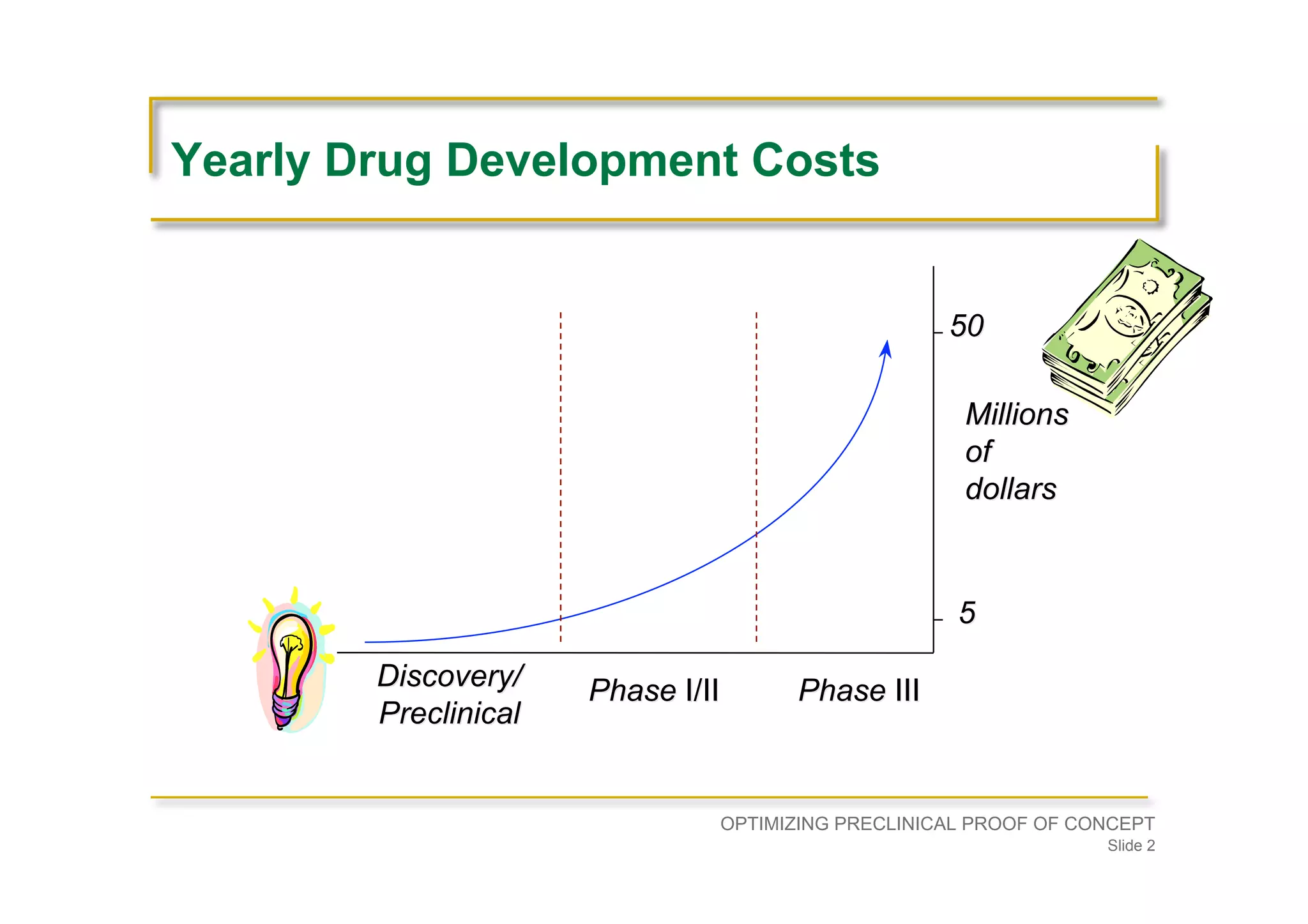
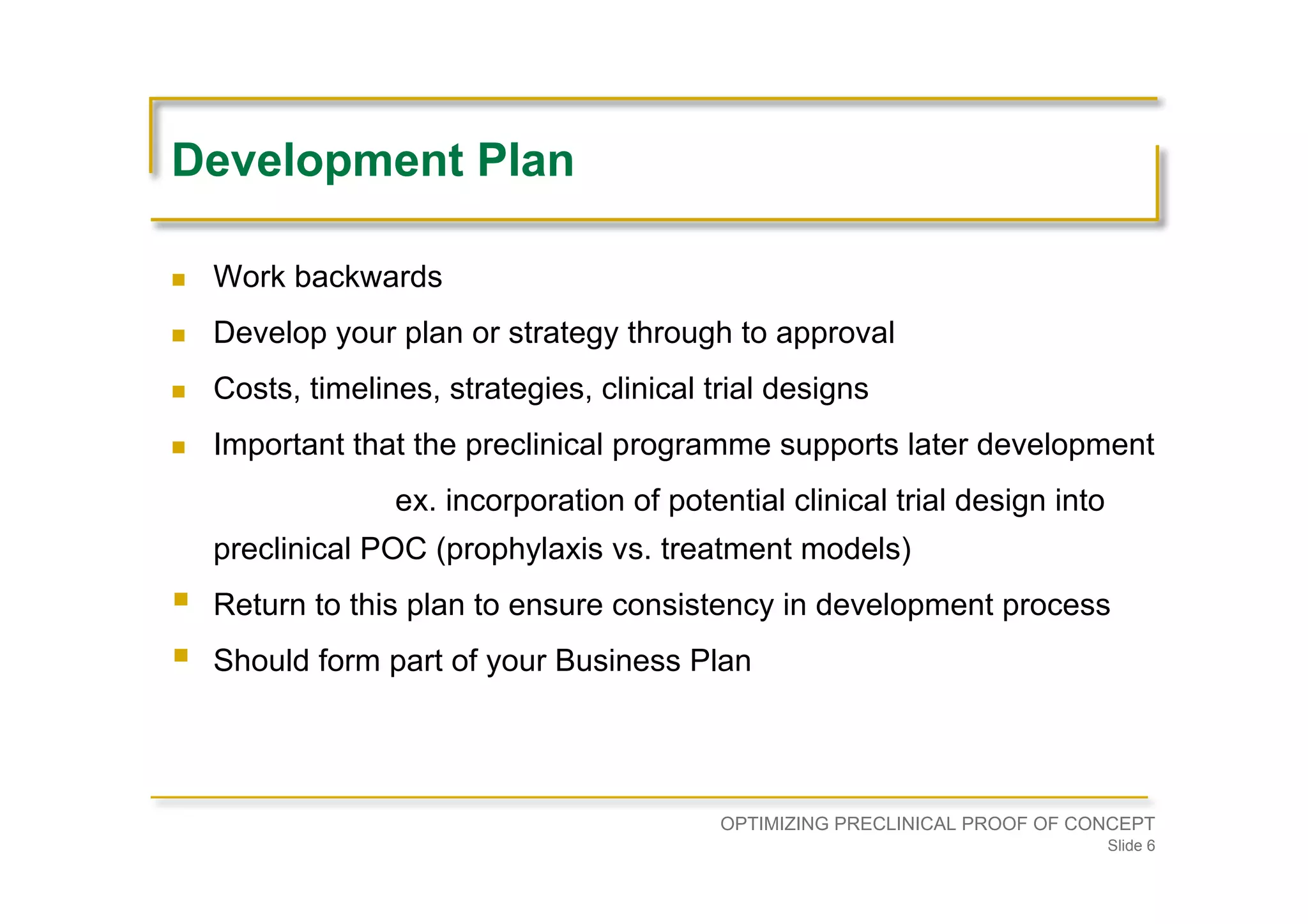
Emphasizes planning backward from approval, ensuring preclinical programs support clinical strategies, including costs and timelines.
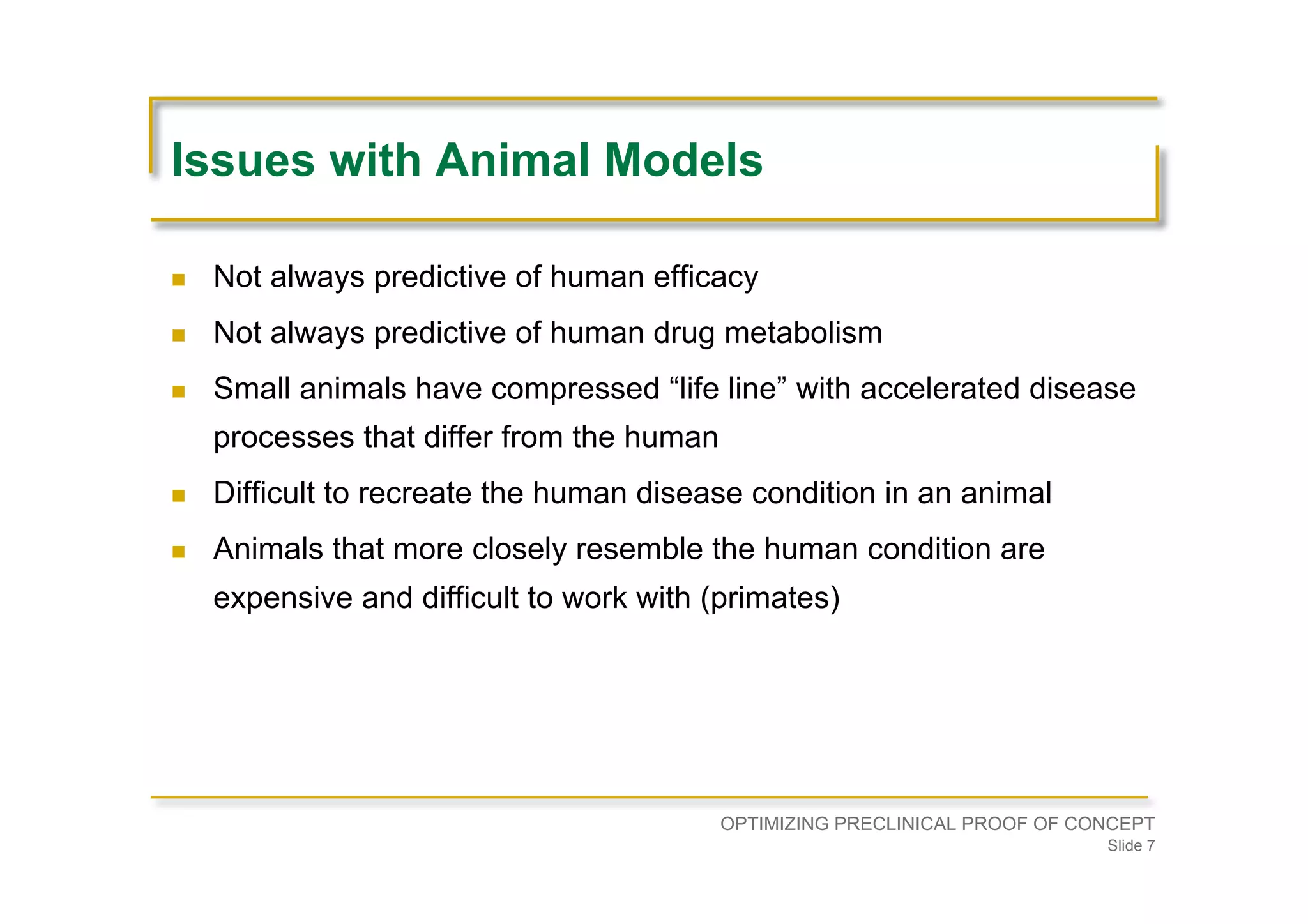
Discusses challenges with animal models in predicting human efficacy and drug metabolism, as well as ethical and practical issues.
Proposes approaches to enhance understanding of mechanism of action (MoA), using relevant animal models and disease mimicking designs.
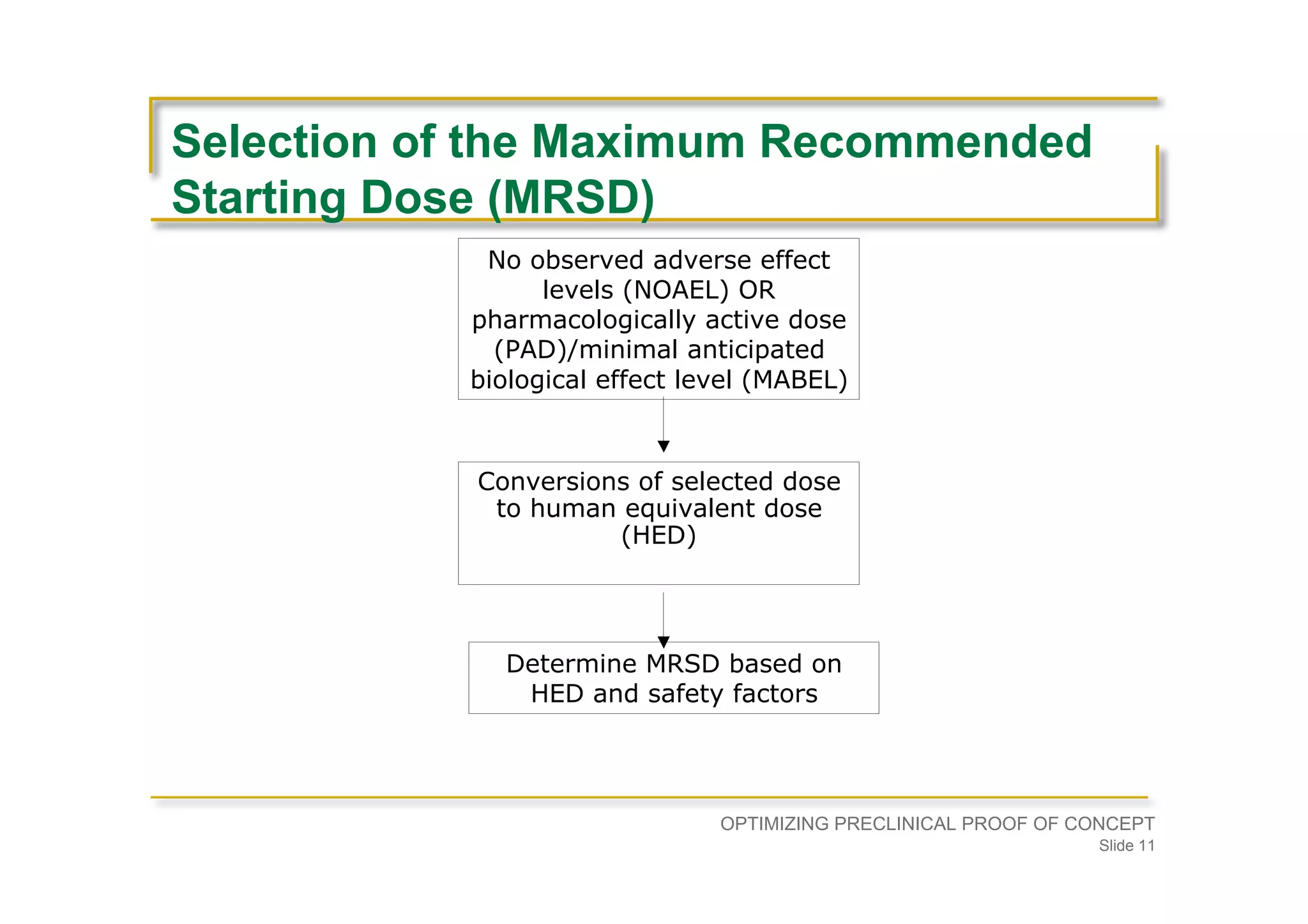
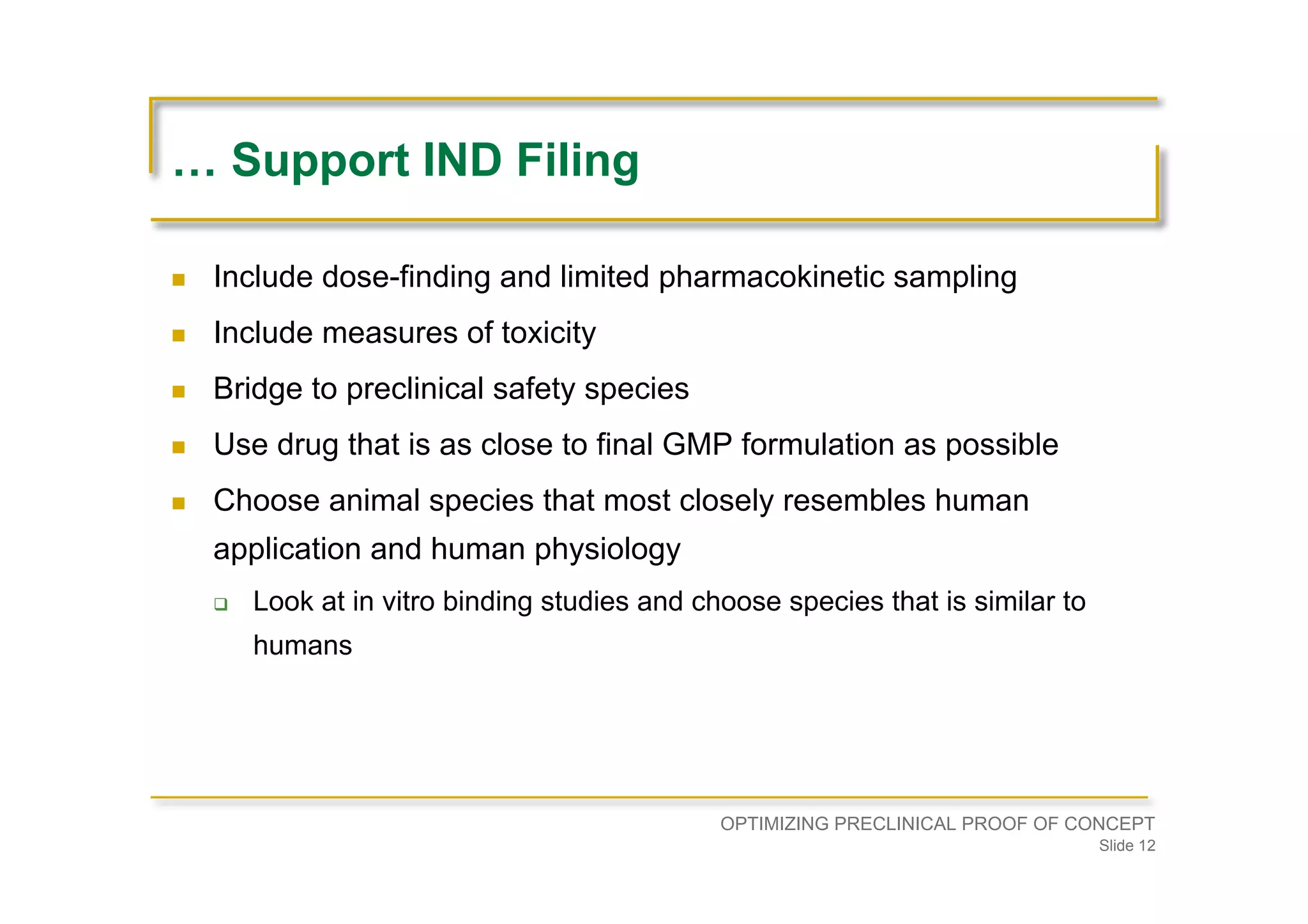
Focuses on the need for physiological evidence in IND filing, addressing dose determination based on NOAEL and HED.
Emphasizes the importance of data quality, including using negative controls, protocol adherence, and GLP standards.
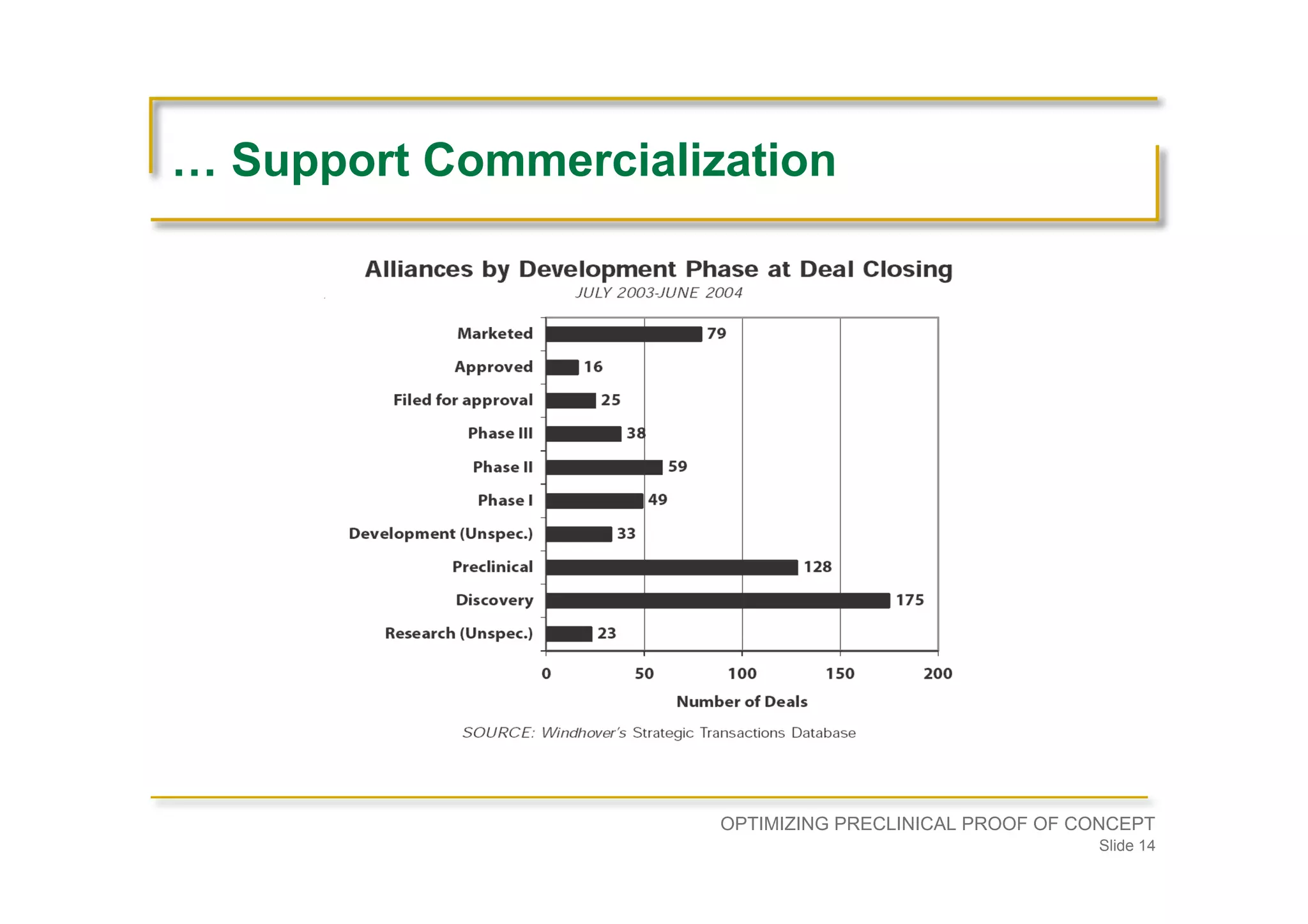
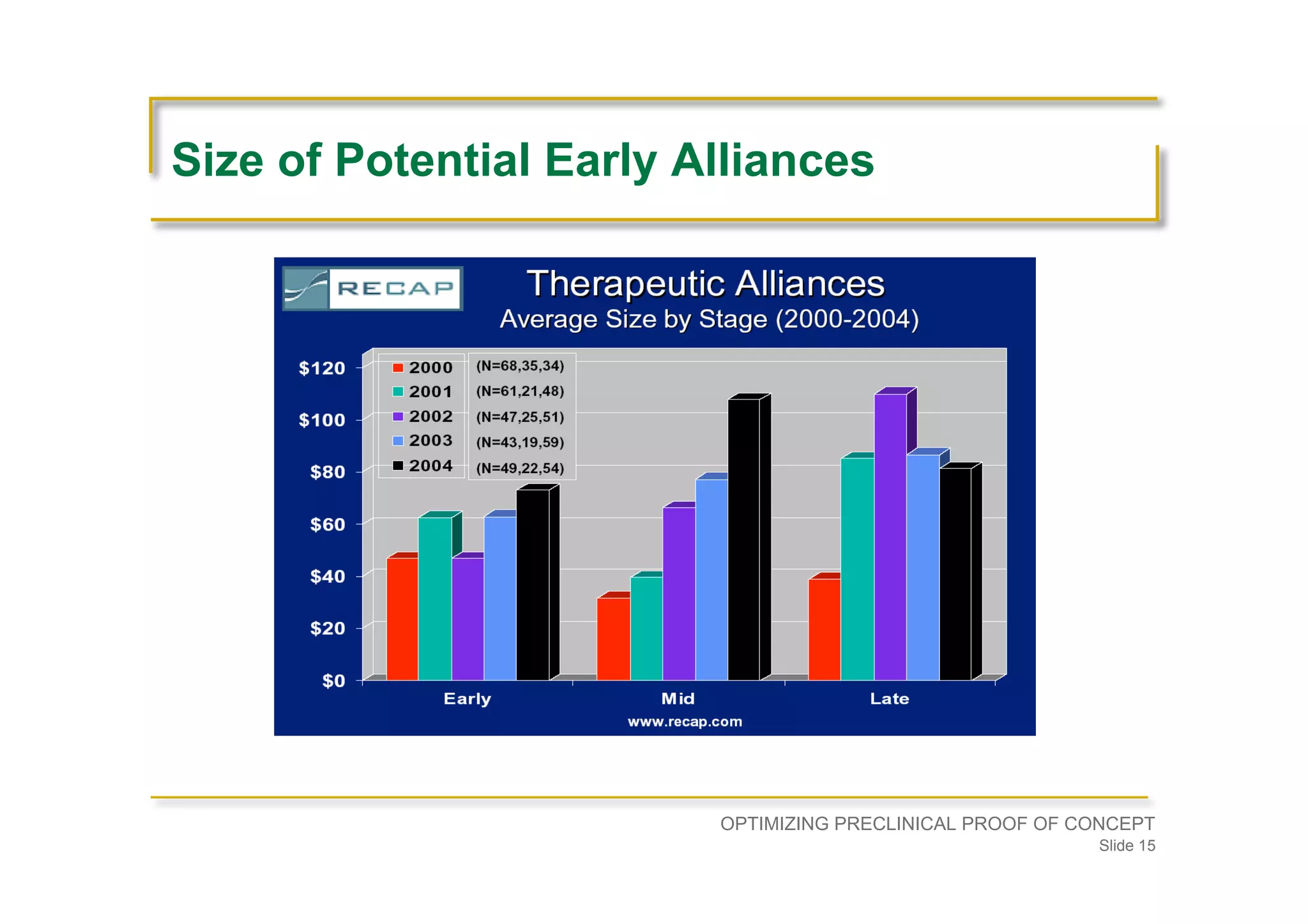
Strategies for partnerships, enhancing patent positions, and ensuring preclinical strategies align with commercial goals.Advises on the establishment of a development plan, understanding disease physiology, and seeking experienced guidance before entering preclinical development.



















