Embed presentation
Download to read offline

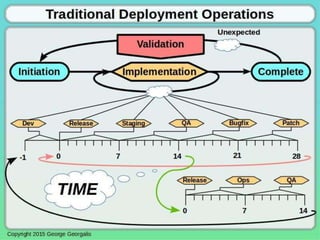
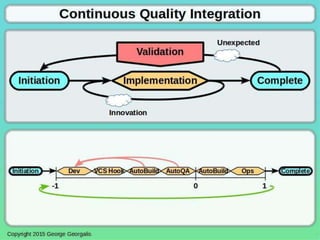

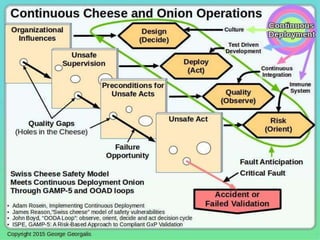

This document discusses applying guidance from pharmaceutical manufacturing processes to DevOps systems development lifecycles to enable agile, continuous operations. It focuses on a risk-based approach to compliance and readiness drawing from good automated manufacturing process guidance for continuous integration and quality assurance.




