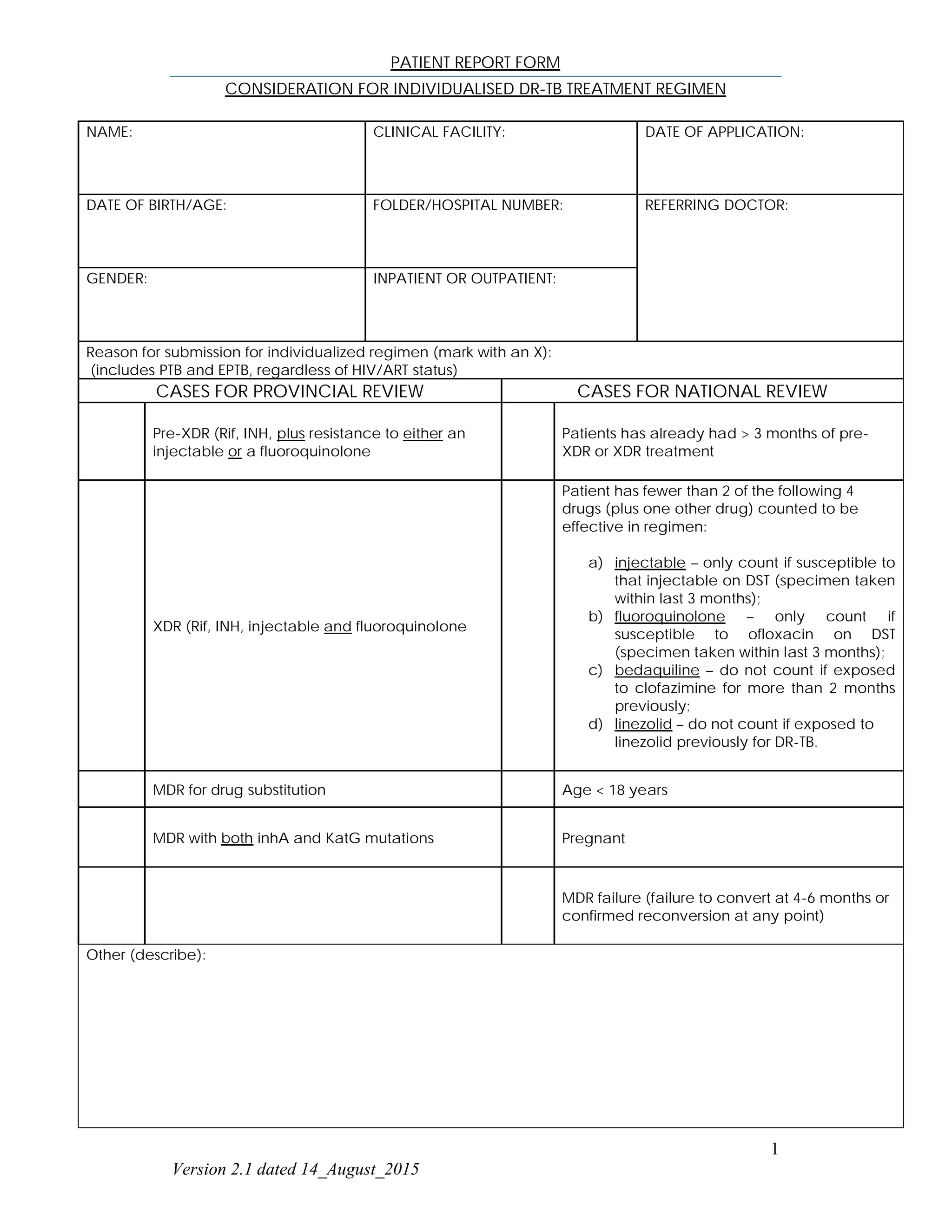
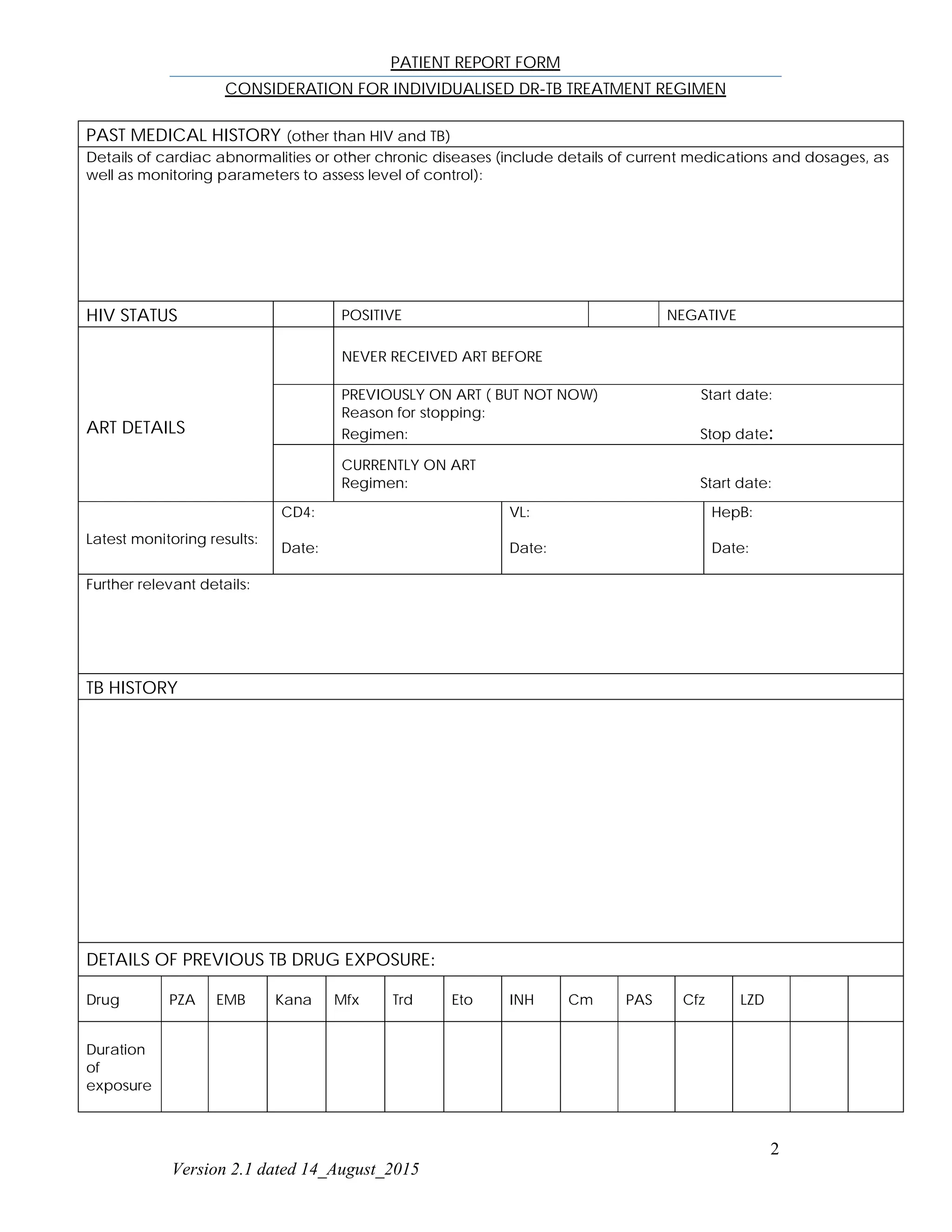
This patient report form documents information for an individual seeking an individualized drug-resistant tuberculosis (DR-TB) treatment regimen. It includes the patient's name, demographic information, reason for submission, past medical history including HIV/ART details, TB history including previous drug exposures, clinical and laboratory findings, proposed DR-TB treatment regimen, and contact information for the responsible medical officer and dispensing pharmacist.