The document discusses the National Pharmaceutical Pricing Authority (NPPA) and the Drug Price Control Order (DPCO) in India. Some key points:
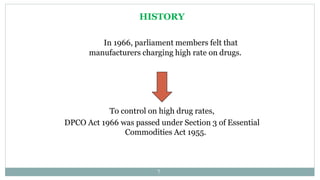
- DPCO allows the government to regulate prices of essential drugs and formulations. It has been amended several times since 1970 to further strengthen price control.
- The NPPA was established in 1997 to implement and monitor the DPCO. It monitors drug prices, availability, and company profitability.
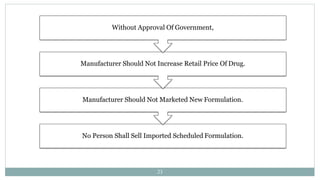
- The DPCO classifies drugs and sets rules for fixing prices of scheduled bulk drugs and formulations based on production costs. It also defines penalties for overcharging.
- The DPCO aims to ensure adequate drug supply at fair prices while allowing companies reasonable