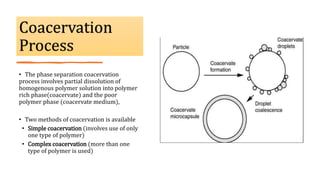
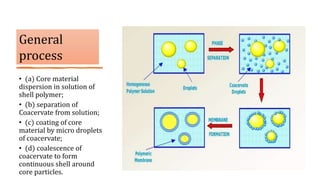
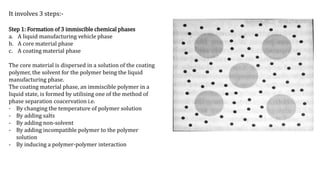
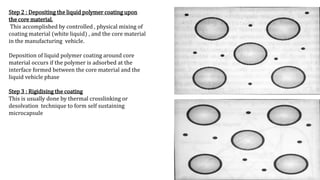
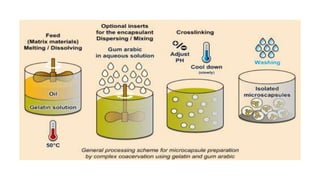
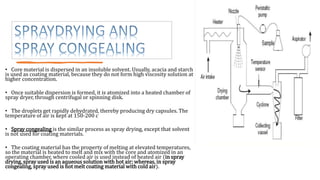
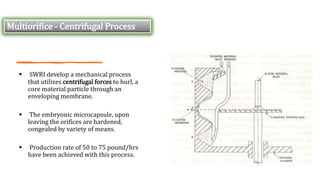
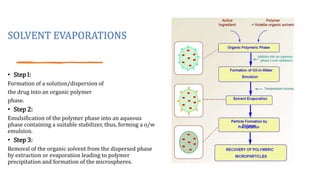
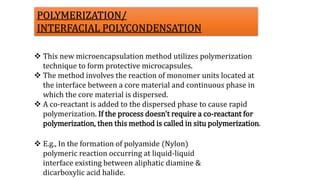
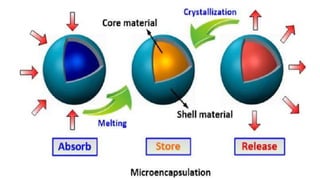
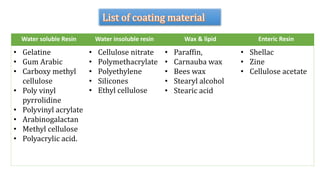
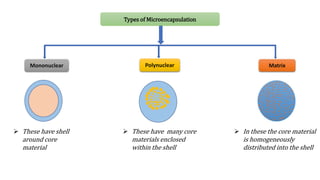
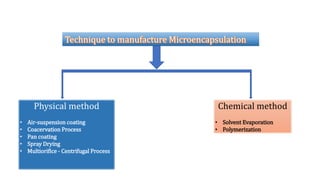
Microencapsulation is the process of enclosing substances, such as solid, liquid, or gas, within microscopic particles using a coating material, yielding capsules of varying sizes. The process involves core materials, typically drugs or additives, and an outer coating of inert substances which can affect drug delivery and stability. Various techniques for microencapsulation include physical methods, such as air-suspension coating and coacervation, as well as chemical methods like solvent evaporation and polymerization.




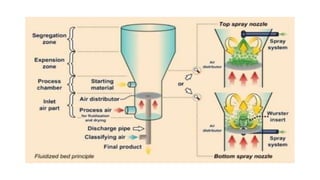
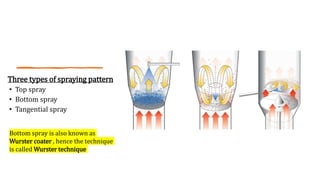
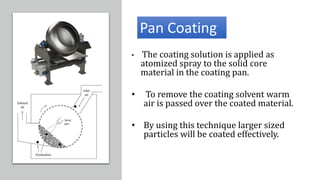
![• This technique is limited to encapsulating solid particle or porous
particle into which liquid has been absorbed.
• The solid particulate core material is suspended in a gas stream
• Usually, heated air ,and a liquid coating material is sprayed on these
air –suspended particles.
• The coated particle are cyclized into zones, where the coating is
dried by solvent evaporation.
• This coating and drying sequence is repeated until the desired
thickness of coating has been applied.
Air-suspension coating
[Fluidized Bed Dryer]](https://image.slidesharecdn.com/microencapsulation-210923165141/85/Microencapsulation-10-320.jpg)