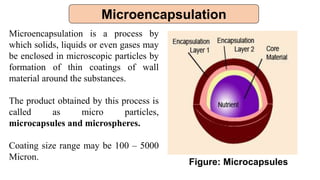
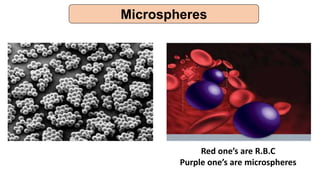
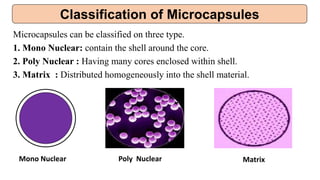
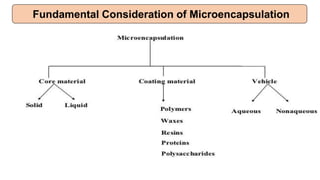
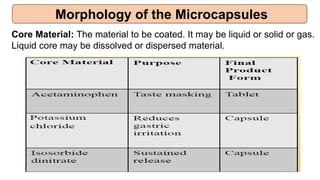
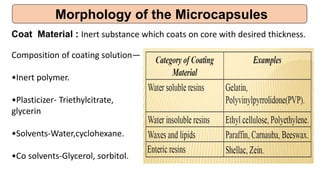
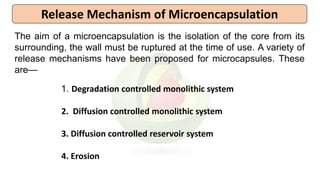
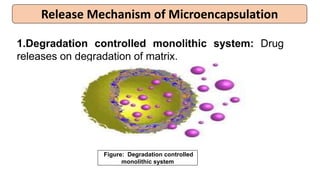
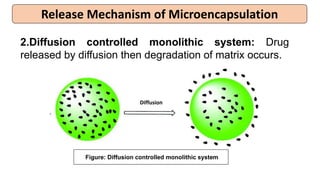
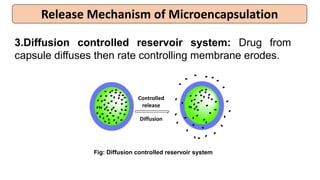
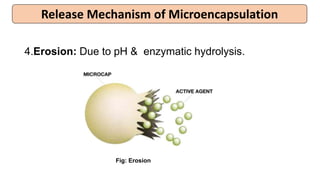
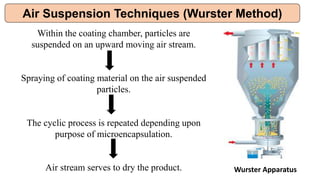
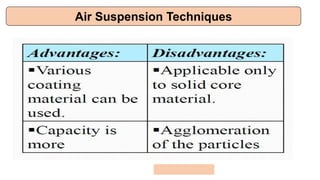
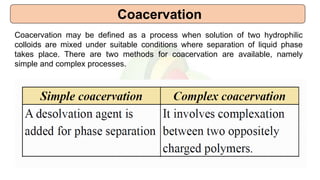
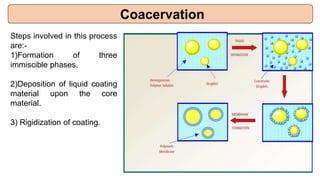
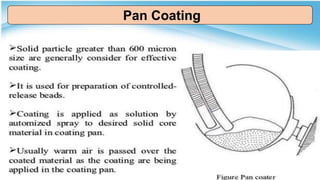
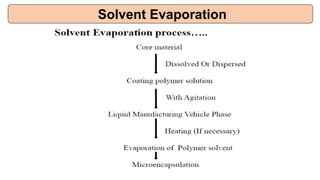
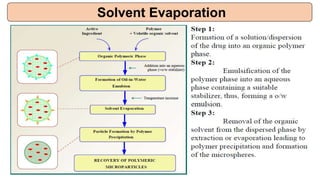
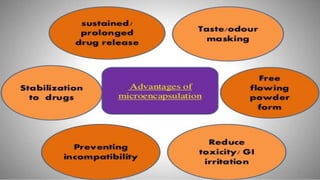
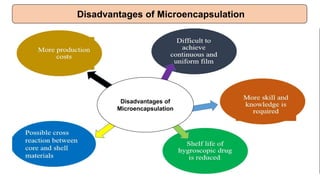
This document provides an overview of microencapsulation including its classification, fundamental considerations, morphology, coating materials, reasons for use, release mechanisms, techniques, evaluation, applications, and disadvantages. Microencapsulation involves enclosing solids, liquids, or gases in microscopic particles with thin coatings to form microparticles, microcapsules, or microspheres ranging from 100-5000 microns. It allows for controlled release, masking of tastes, and protection of unstable or volatile materials. Common techniques include coacervation, pan coating, spray drying, solvent evaporation, and polymerization.