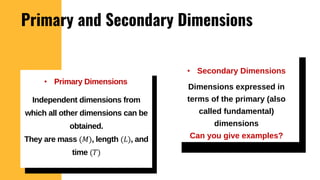
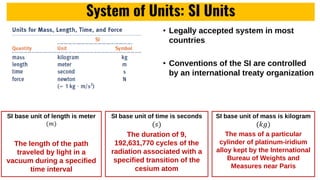
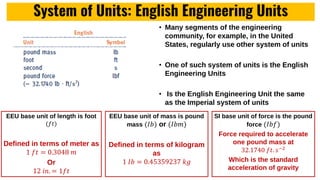
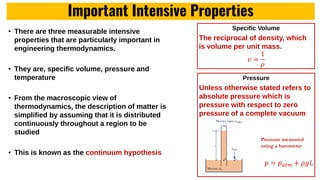
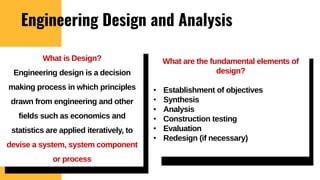
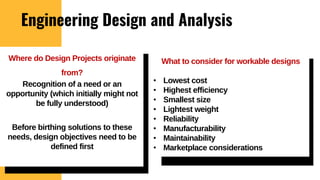
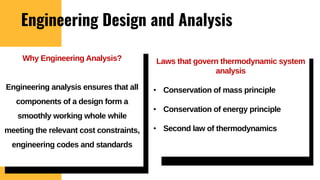
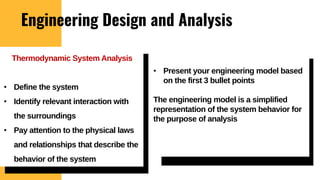
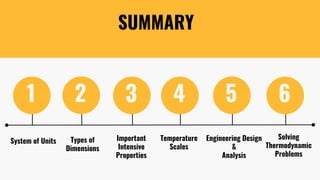
The lecture covers the importance of systems of units in engineering design and analysis, focusing on primary and secondary dimensions, as well as intensive properties in thermodynamics, including specific volume, pressure, and temperature. It also outlines the engineering design process, emphasizing factors like cost, efficiency, and maintainability, alongside methodologies for solving thermodynamic problems. Key temperature scales, such as Kelvin, Celsius, and Rankine, are discussed, and principles governing thermodynamic analysis are highlighted.