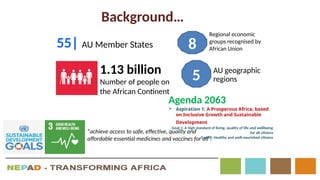
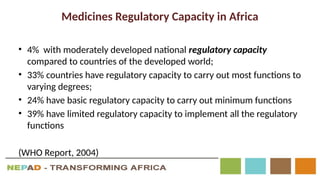
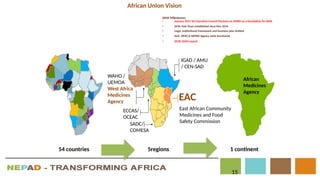
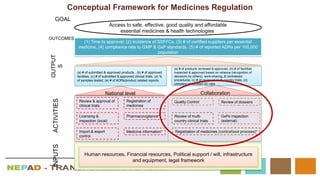
The document outlines the African Medicines Regulatory Harmonization (AMRH) and the establishment of the African Medicines Agency (AMA) as part of the New Partnership for Africa's Development (NEPAD). It discusses the goals of improving access to quality medical products through a harmonized regulatory framework across African nations, addressing the fragmented regulatory systems and inadequate local manufacturing capacity. The AMA aims to enhance the safety and effectiveness of medications within the continent by promoting standards, providing regulatory support, and fostering collaboration among various stakeholders.