The document contains data from multiple studies comparing different wound dressings and skin treatments:
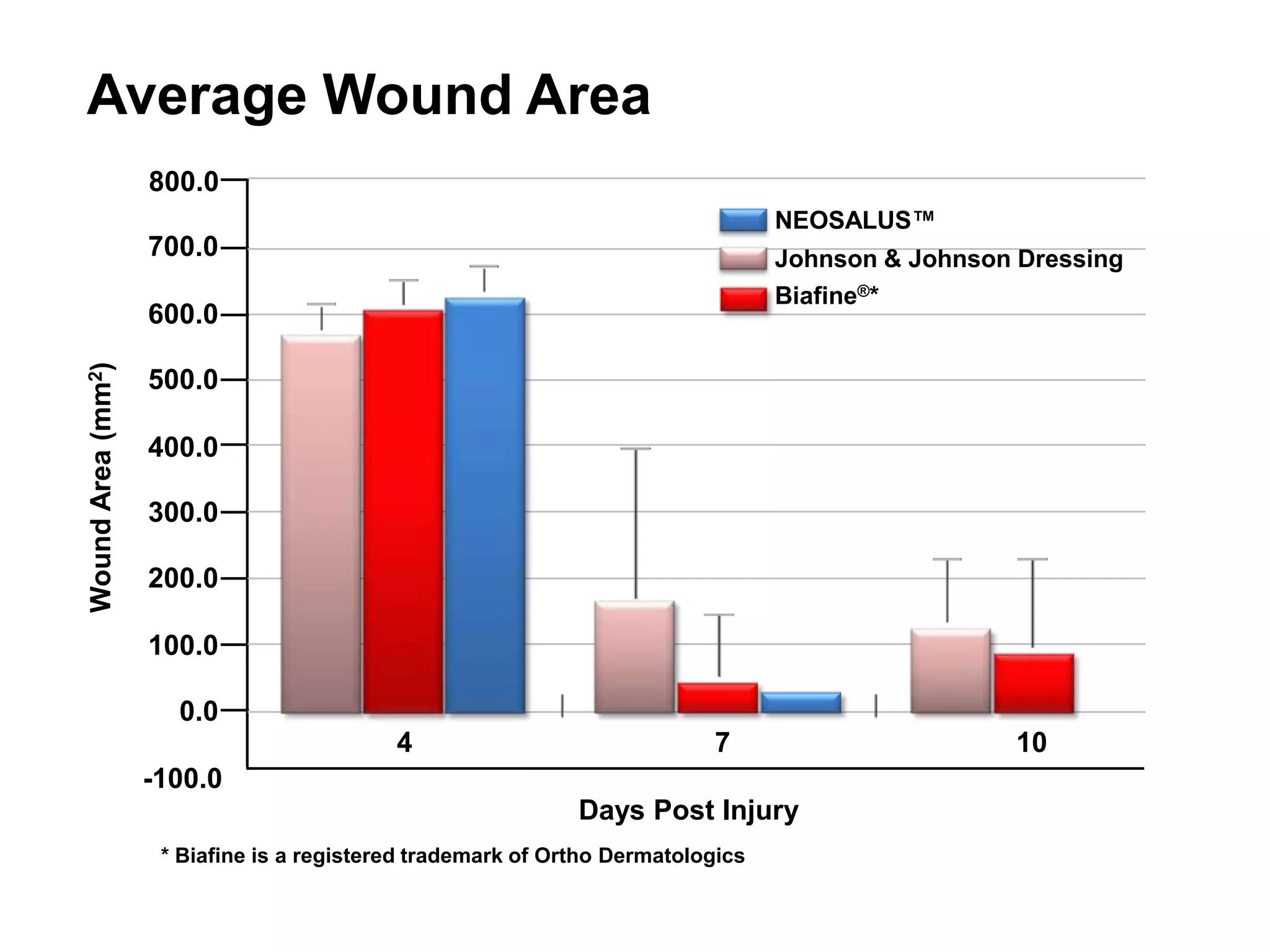
- A graph shows that a NEOSALUSTM dressing reduced wound area more than two other dressings over 10 days.
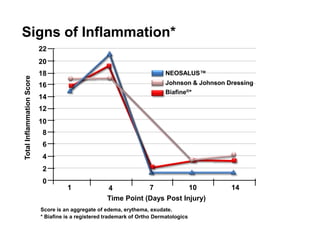
- A table shows that NEOSALUSTM and another dressing had lower inflammation scores than a third dressing at several time points over 14 days.
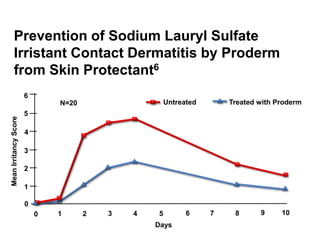
- A graph demonstrates that a skin protectant reduced irritation from sodium lauryl sulfate compared to an untreated control over 10 days.
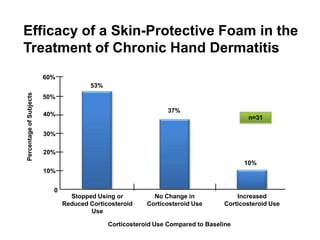
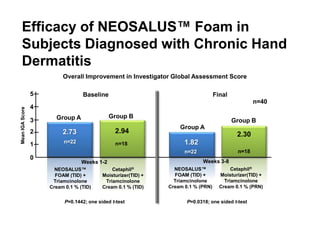
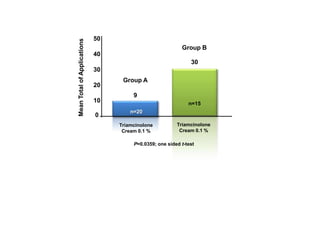
- Two charts indicate that a skin-protective foam reduced corticosteroid use and improved symptoms in patients with chronic hand dermatitis compared to a moisturizer alone.
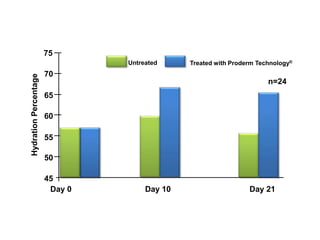
- Additional data and