Embed presentation
Download to read offline



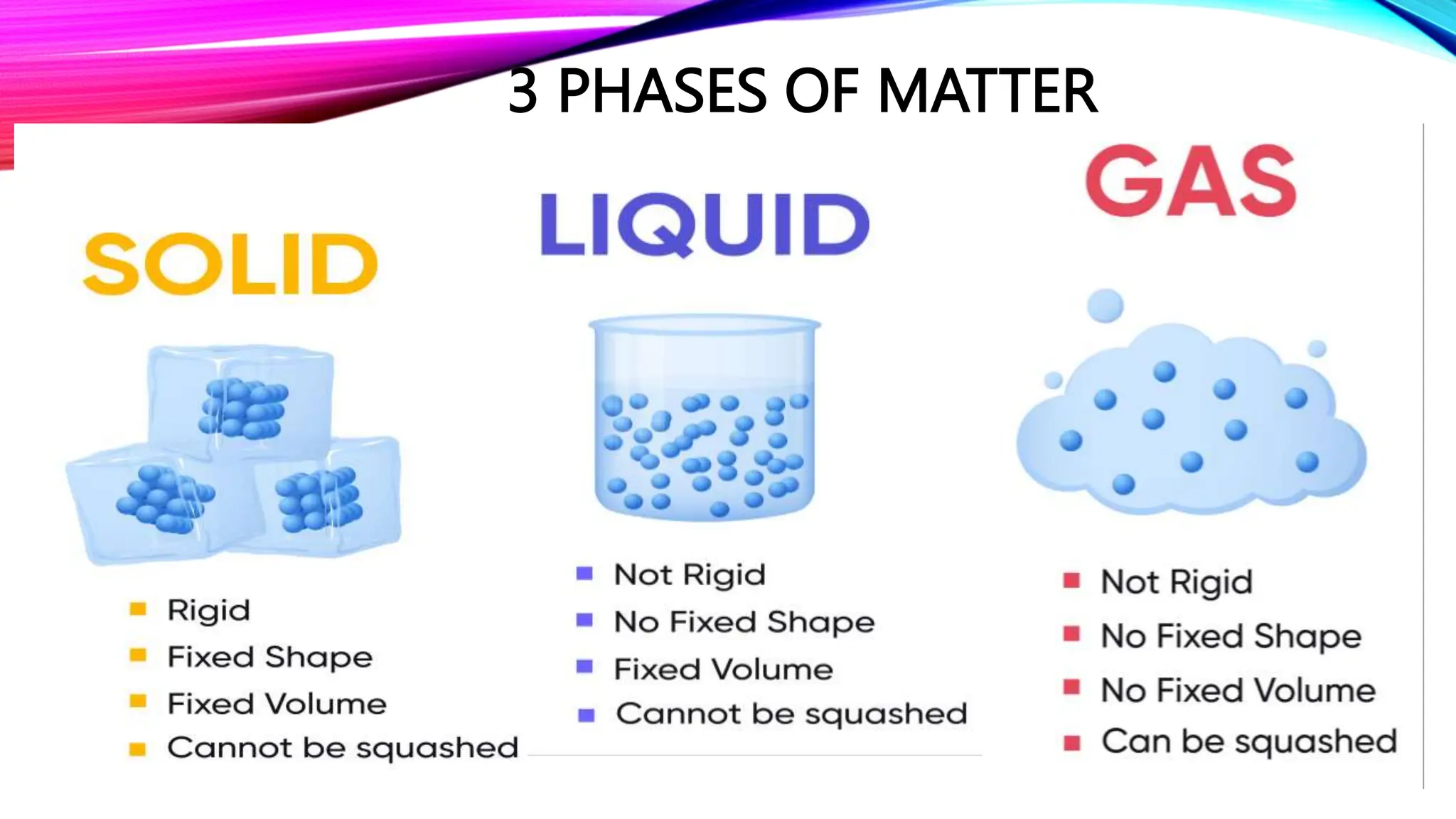

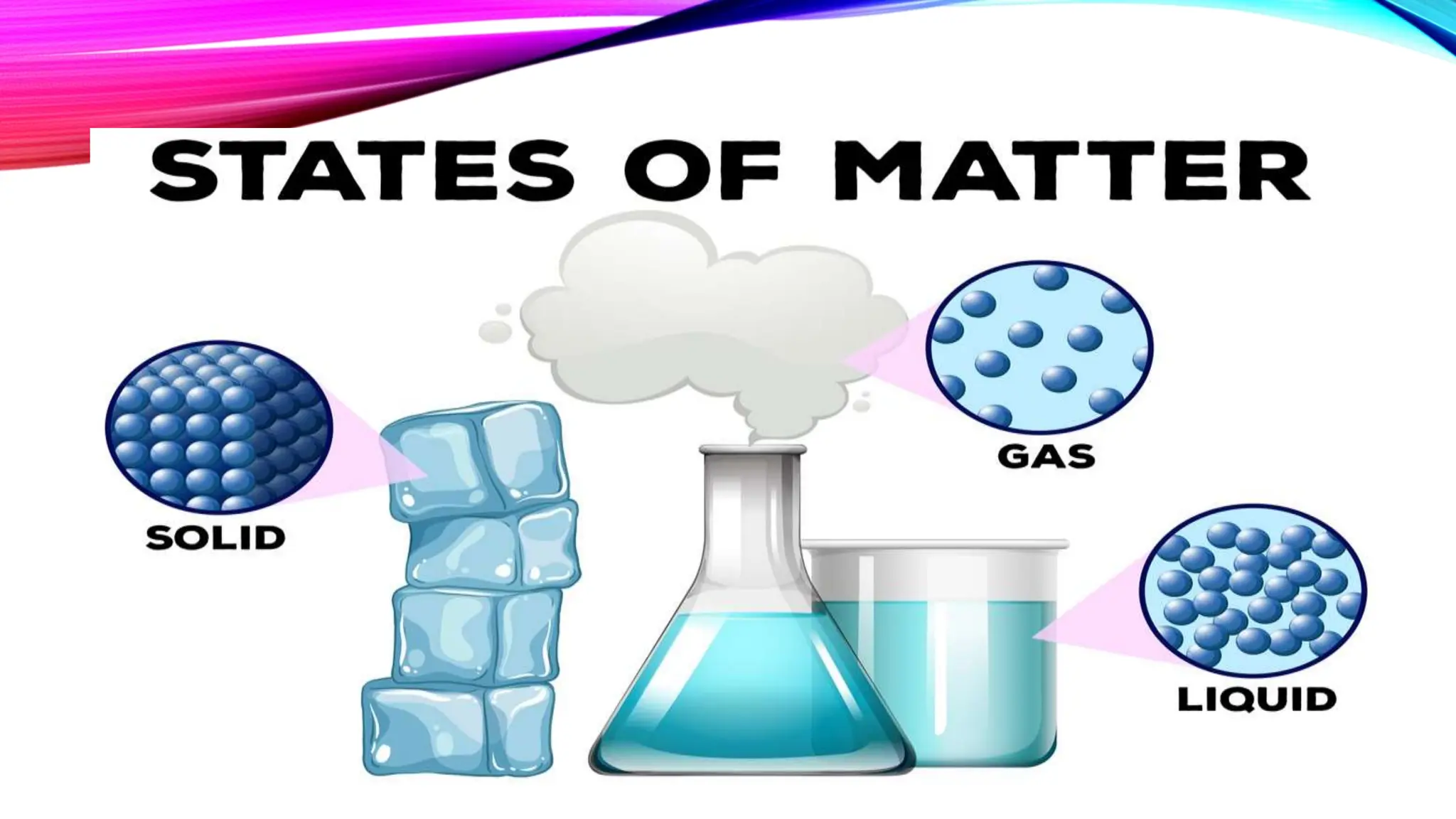
This lesson discusses the three common phases of matter - solids, liquids, and gases - and how their arrangement and properties differ. It explains that solids have a defined shape and volume while liquids and gases can flow and be compressed, and describes the energy required for phase changes between states as molecules gain or lose kinetic energy. Students will learn to apply the particulate nature of matter to explain phases and changes in terms of atomic and molecular arrangement and motion.






