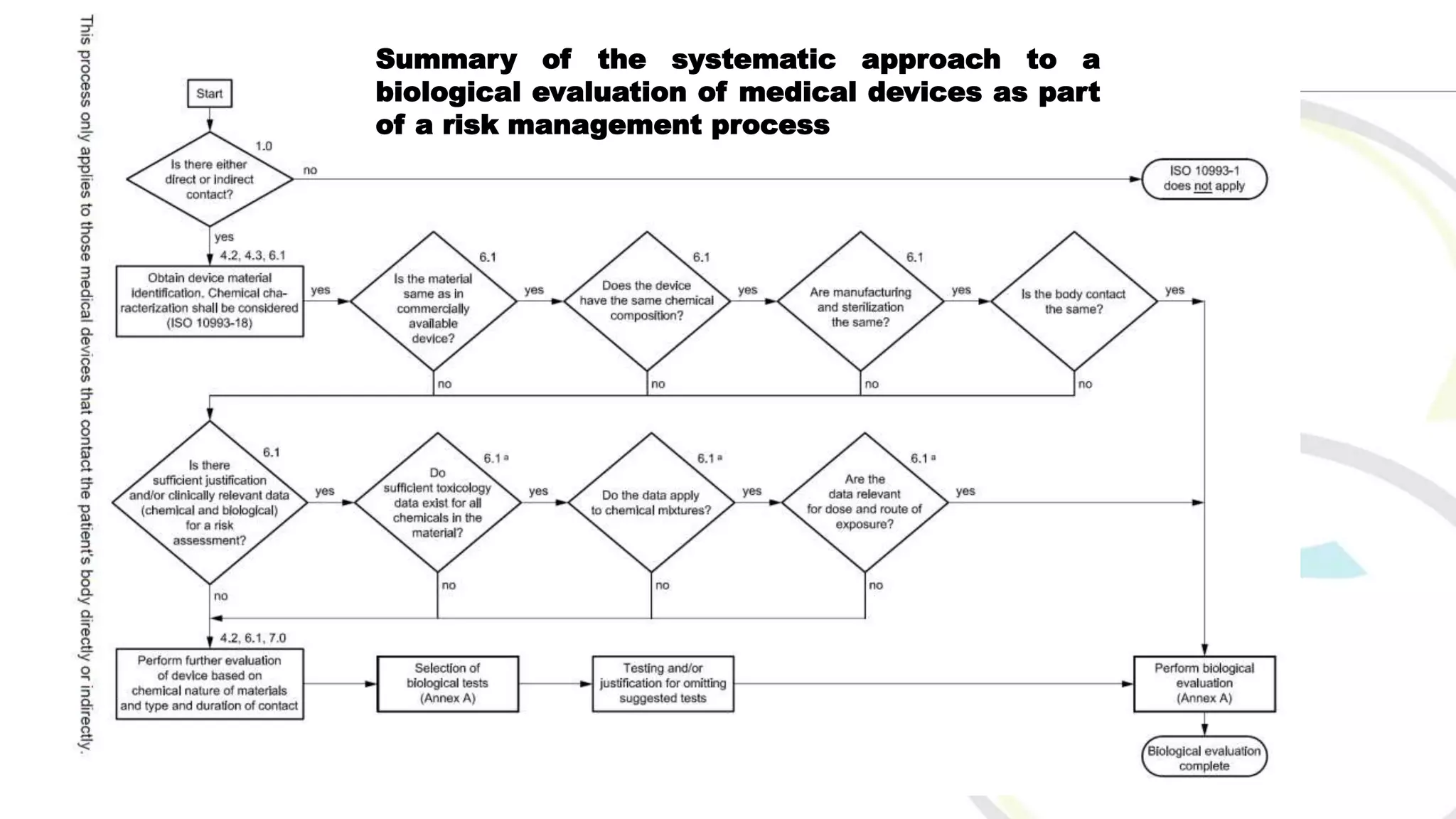
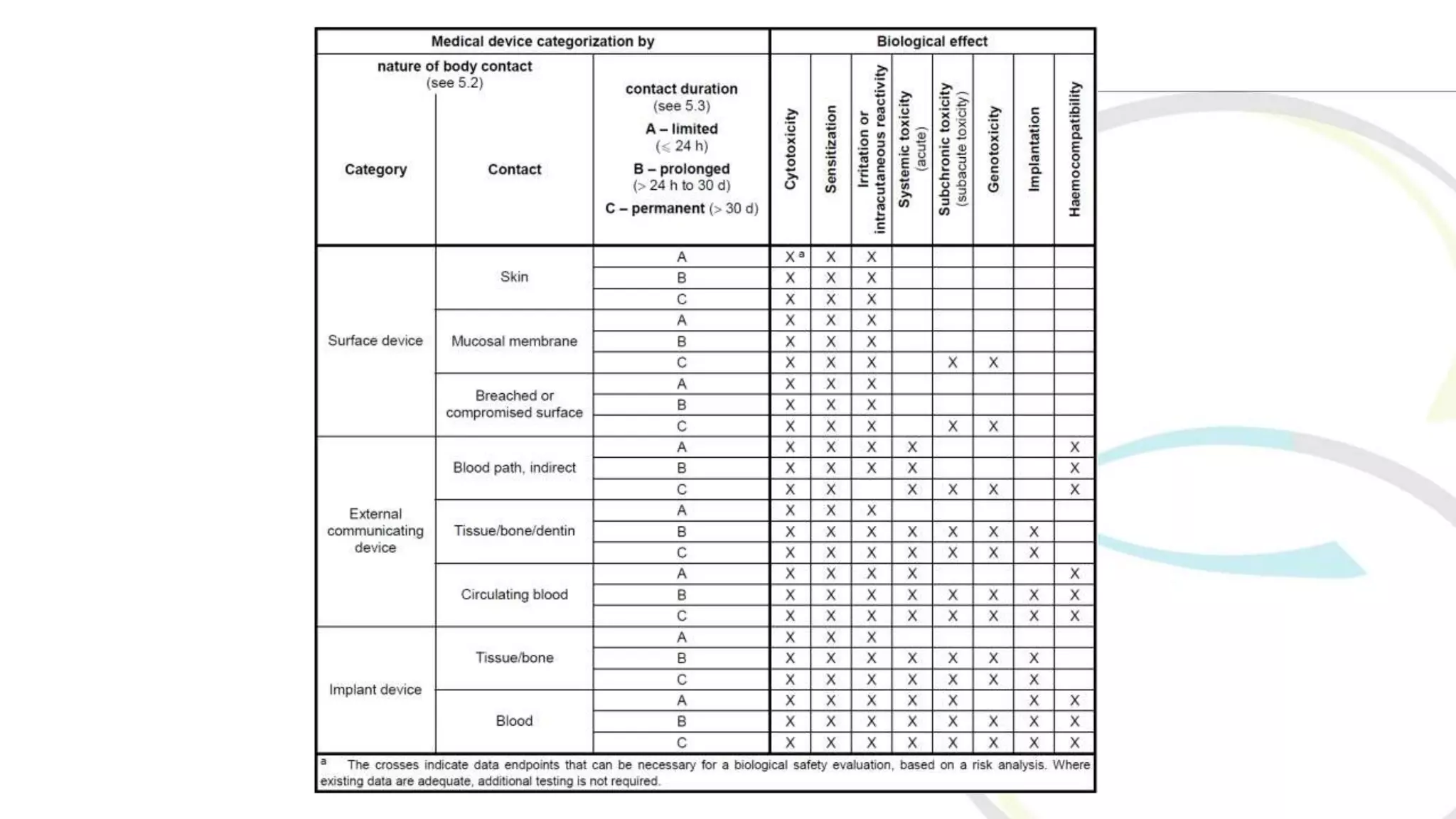
ISO 10993 aims to protect humans from biological risks associated with medical devices through a comprehensive evaluation and testing framework. It covers various aspects, including tests for genotoxicity, cytotoxicity, and systemic toxicity, along with establishing limits for leachable substances. The document emphasizes the importance of selecting appropriate materials and creating a biological evaluation report in the risk management process.