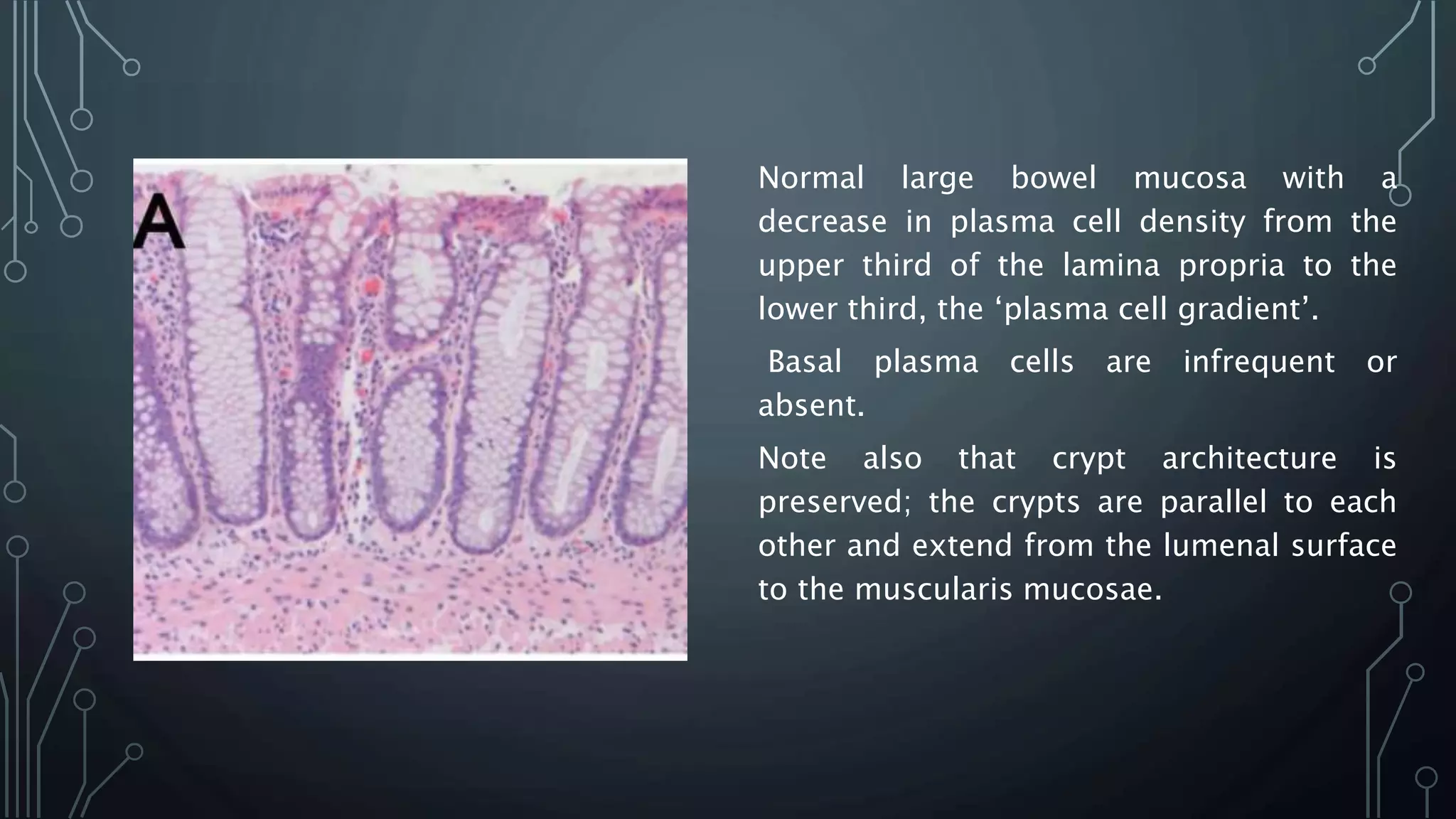
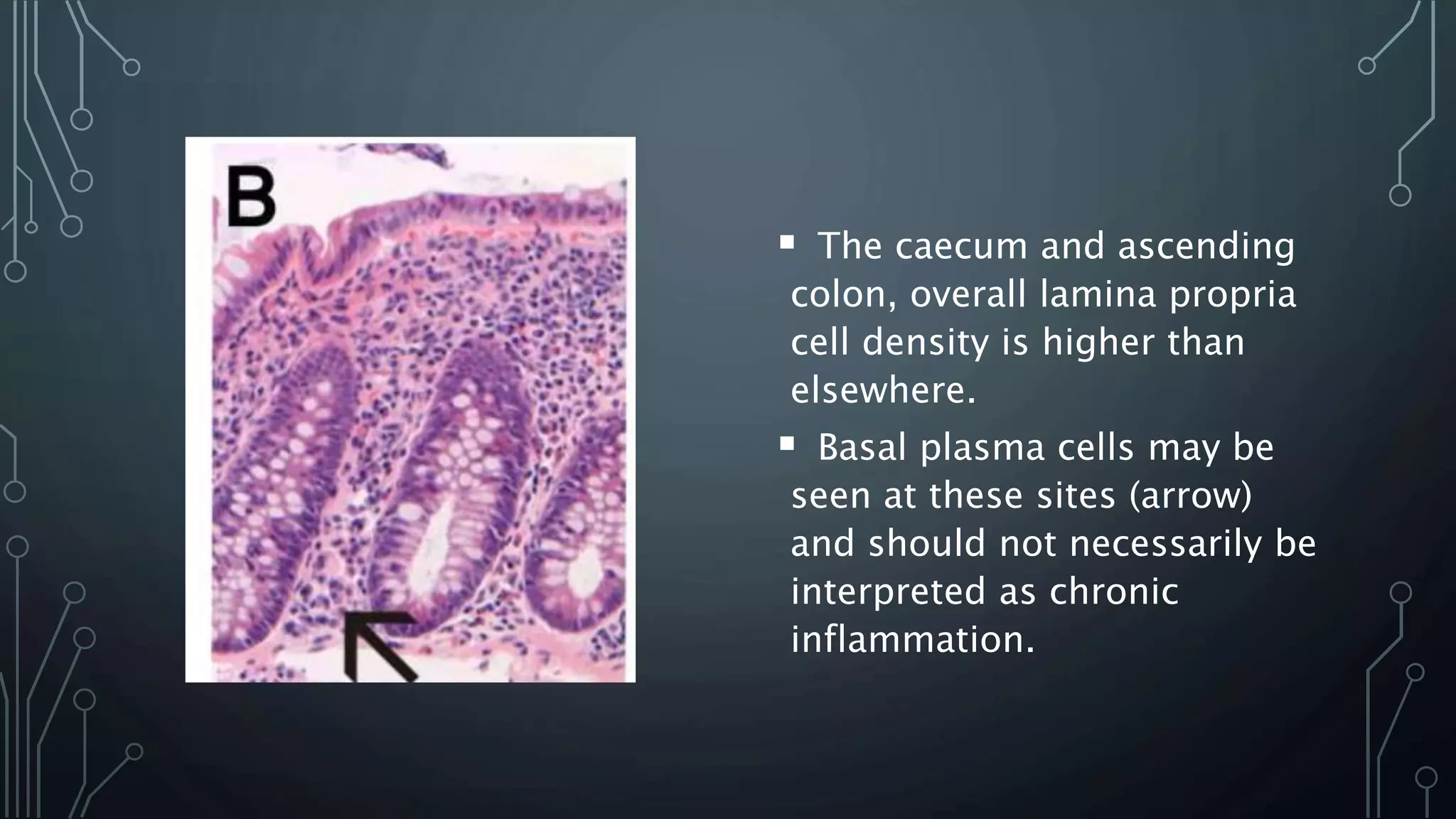
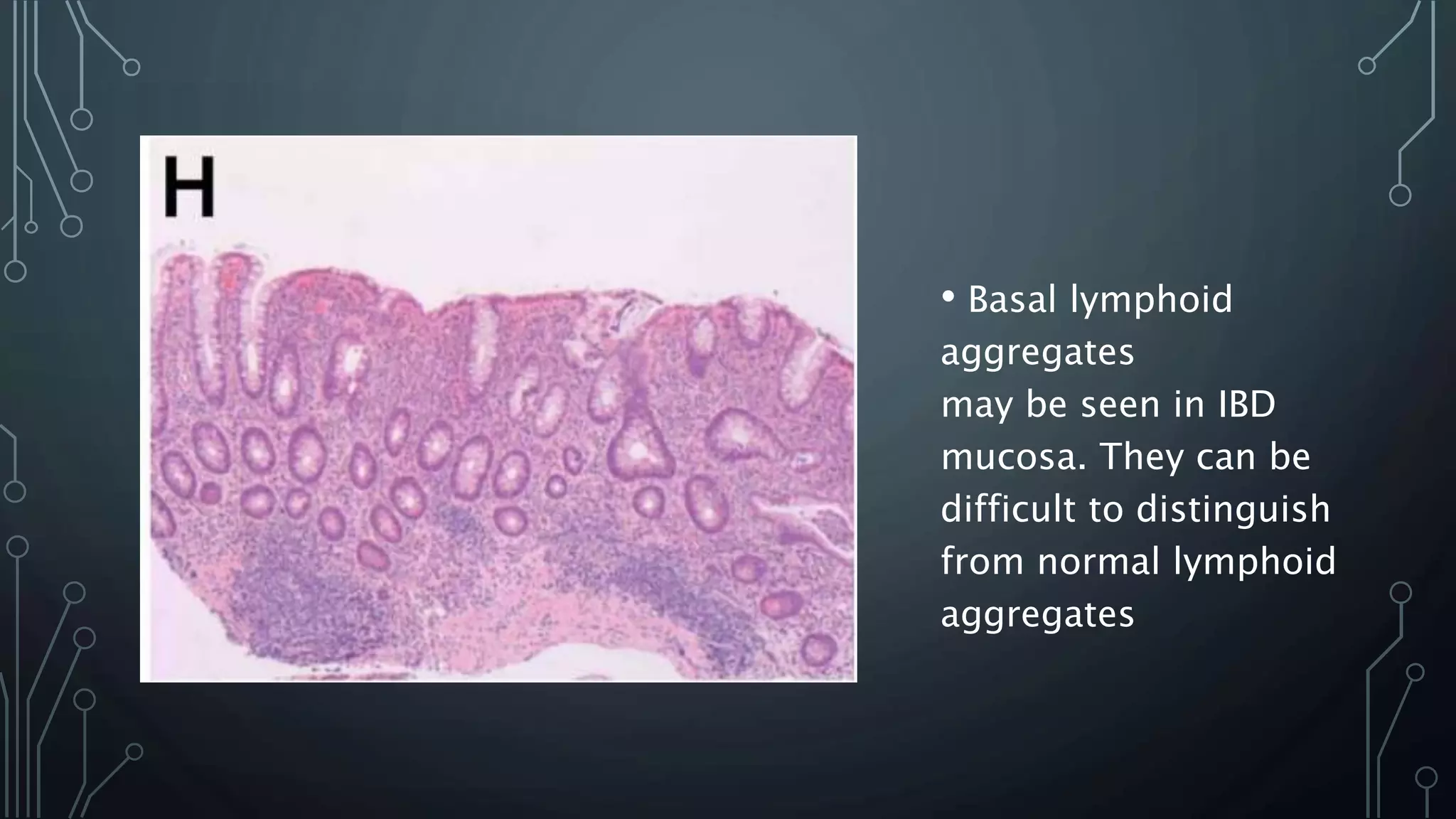
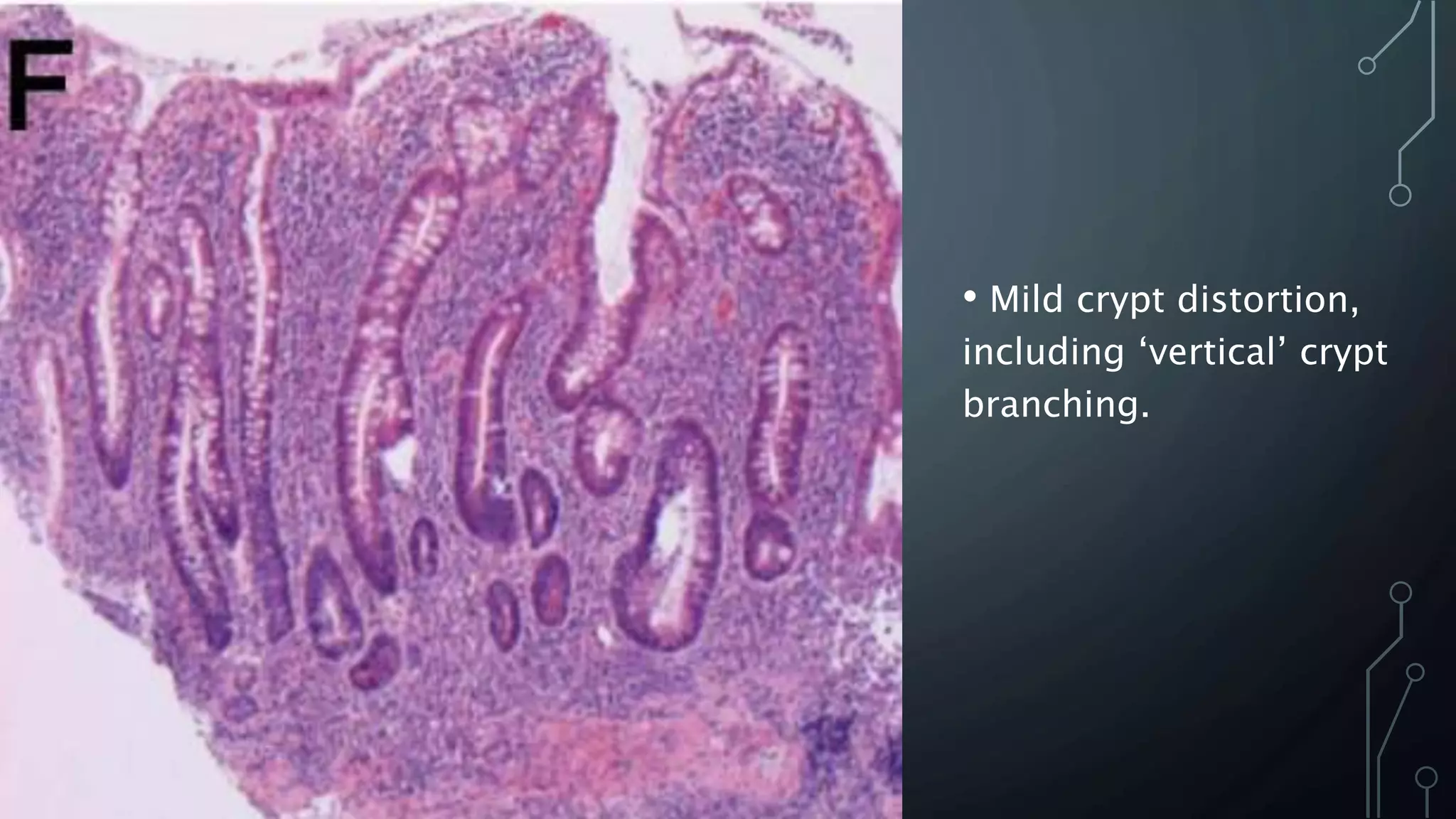
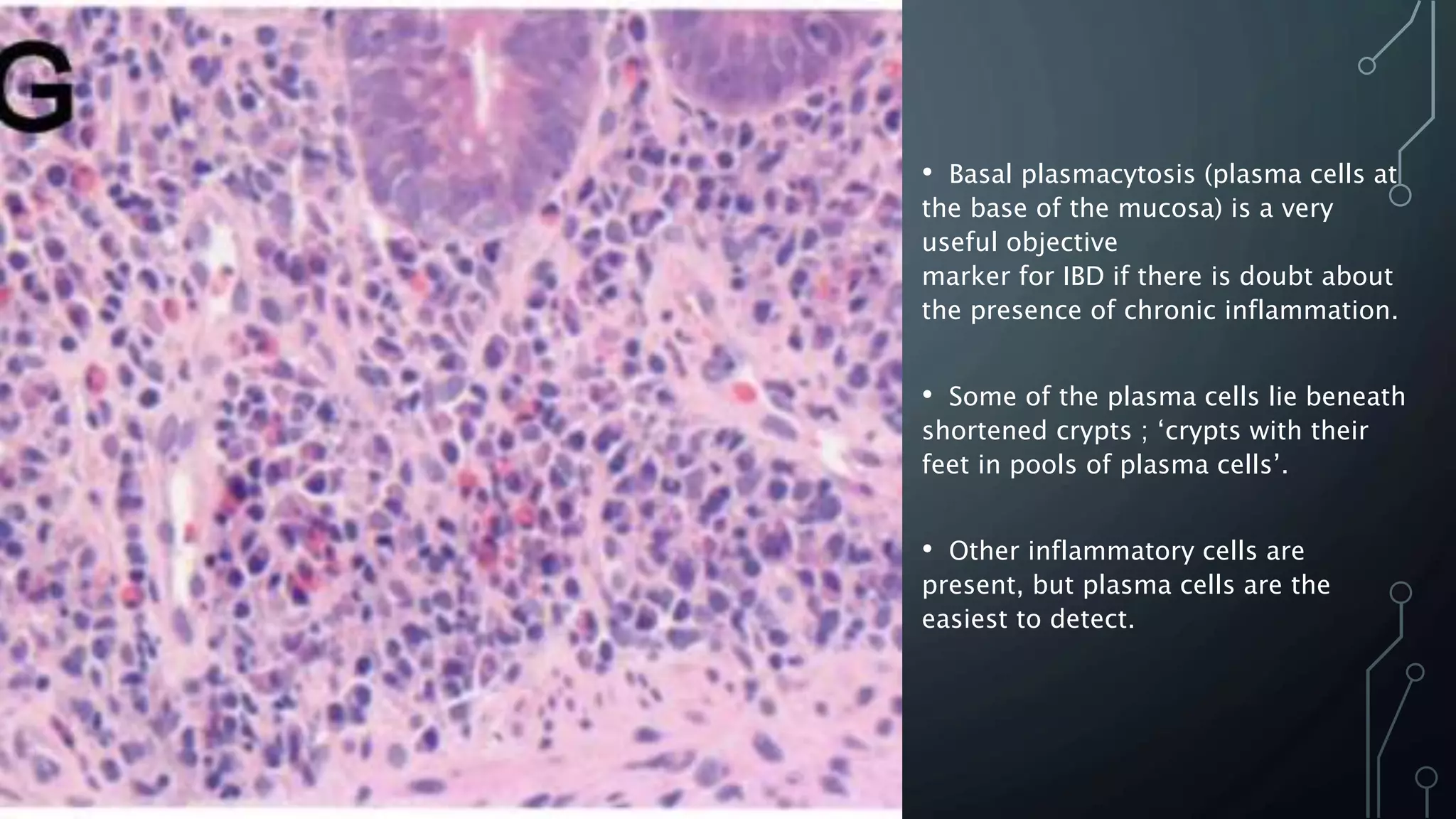
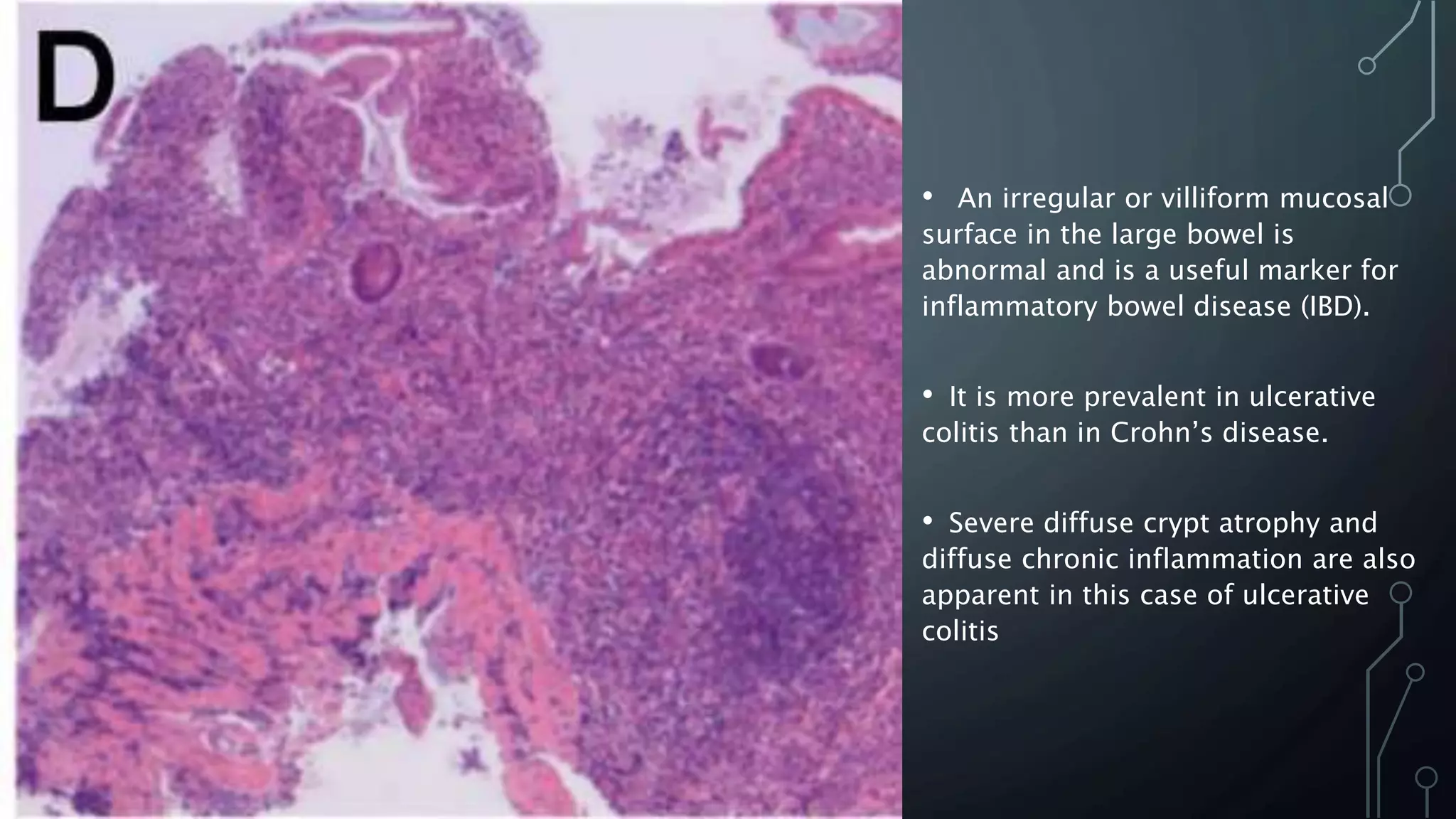
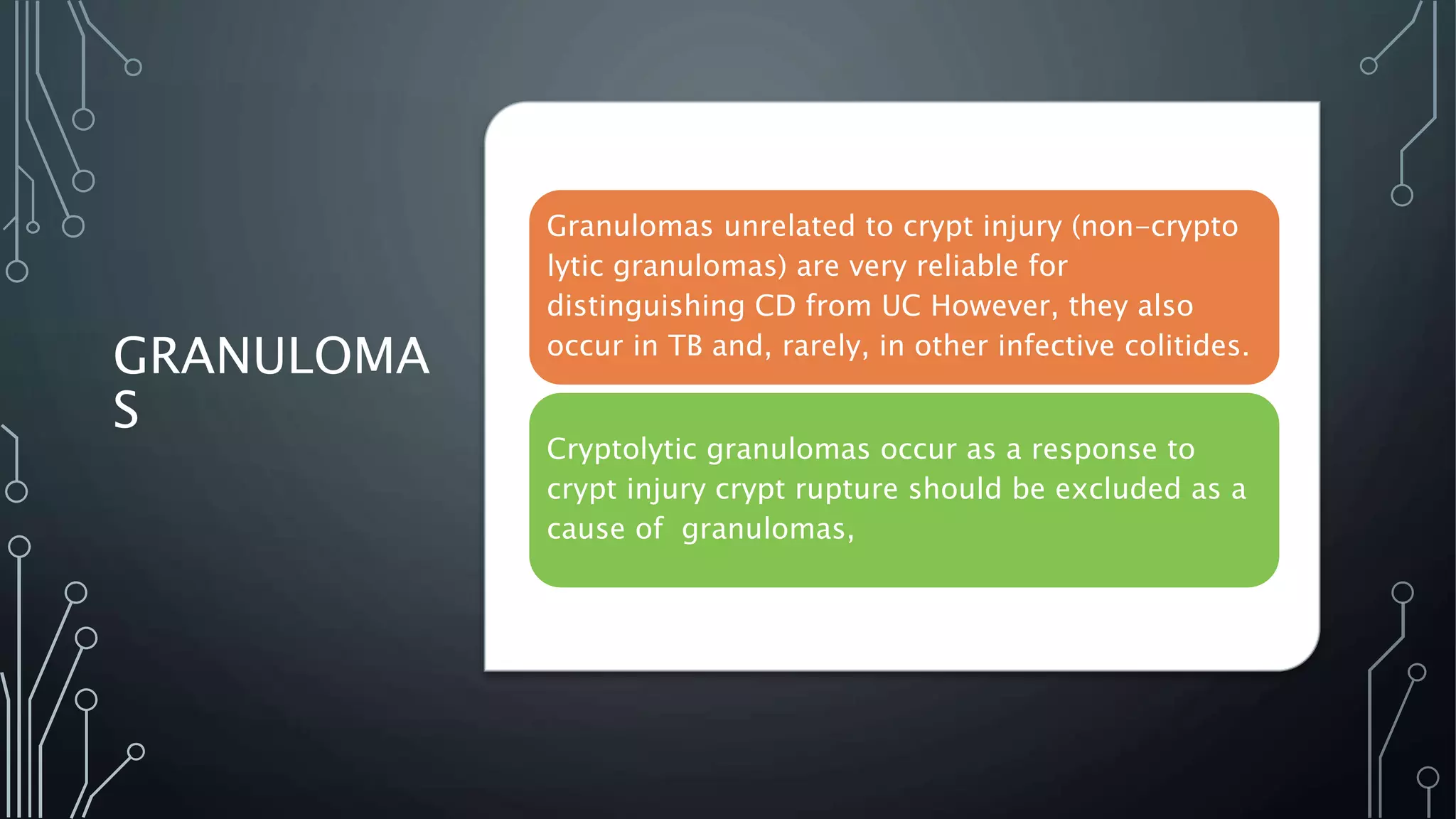
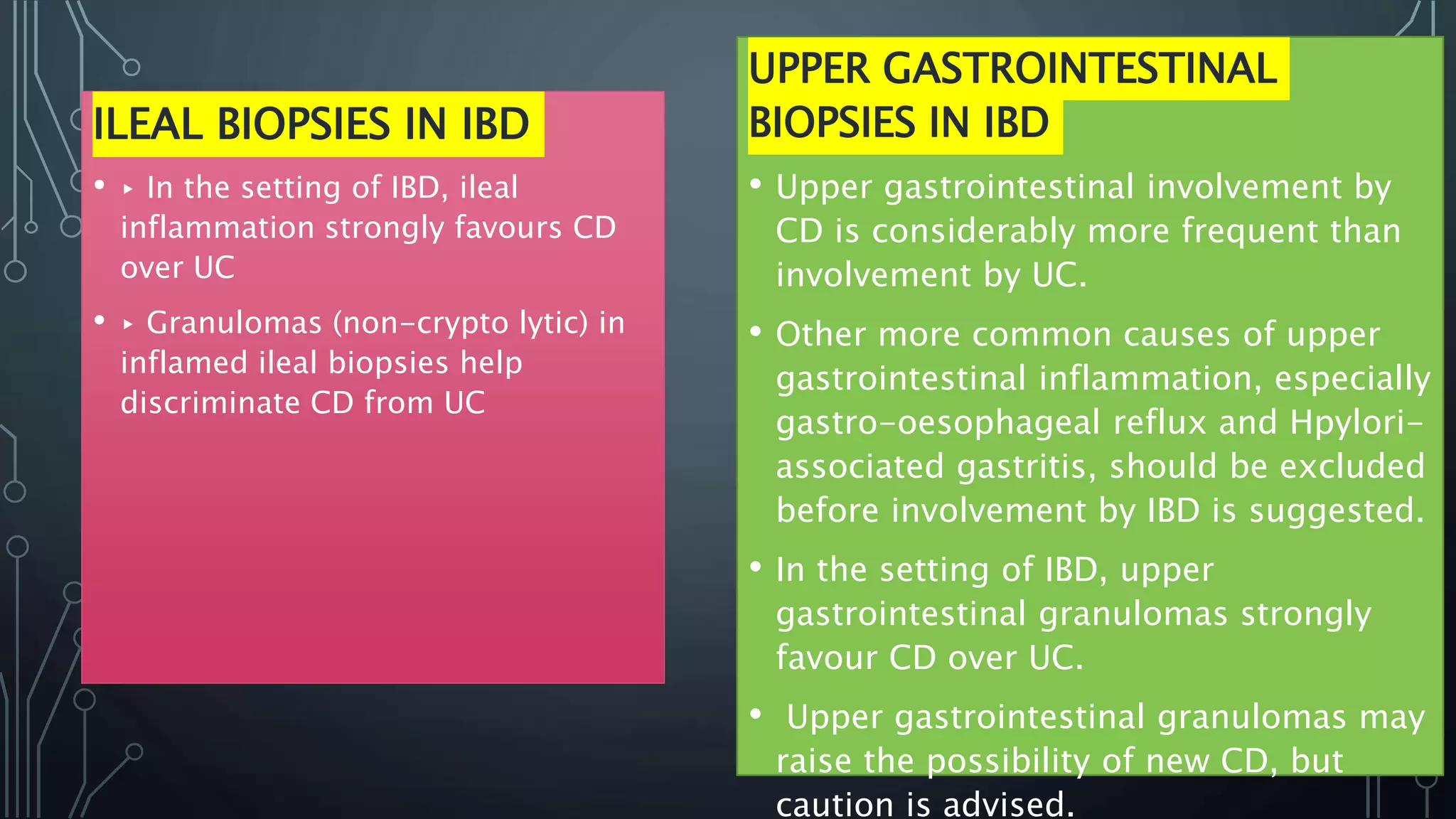
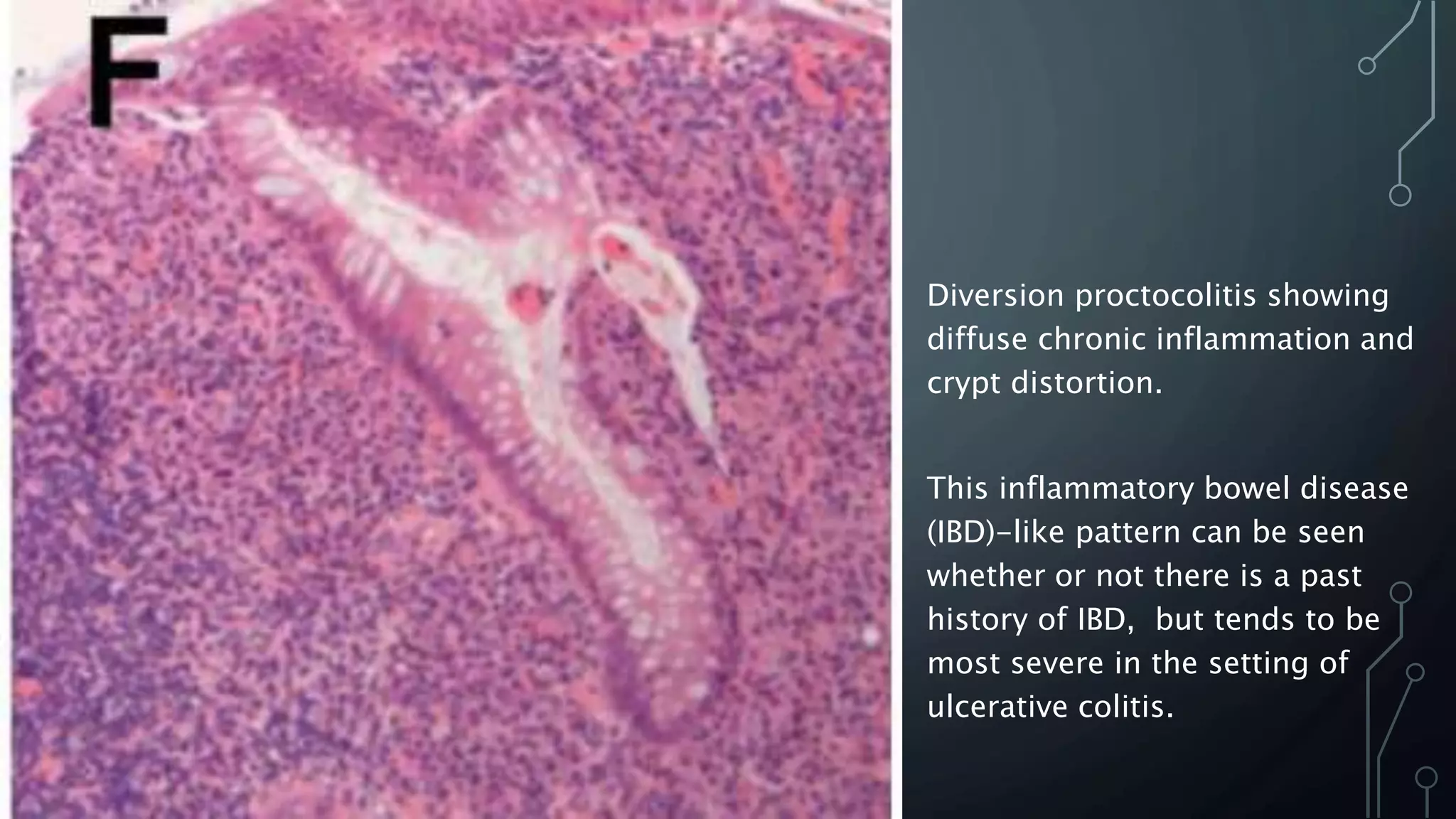
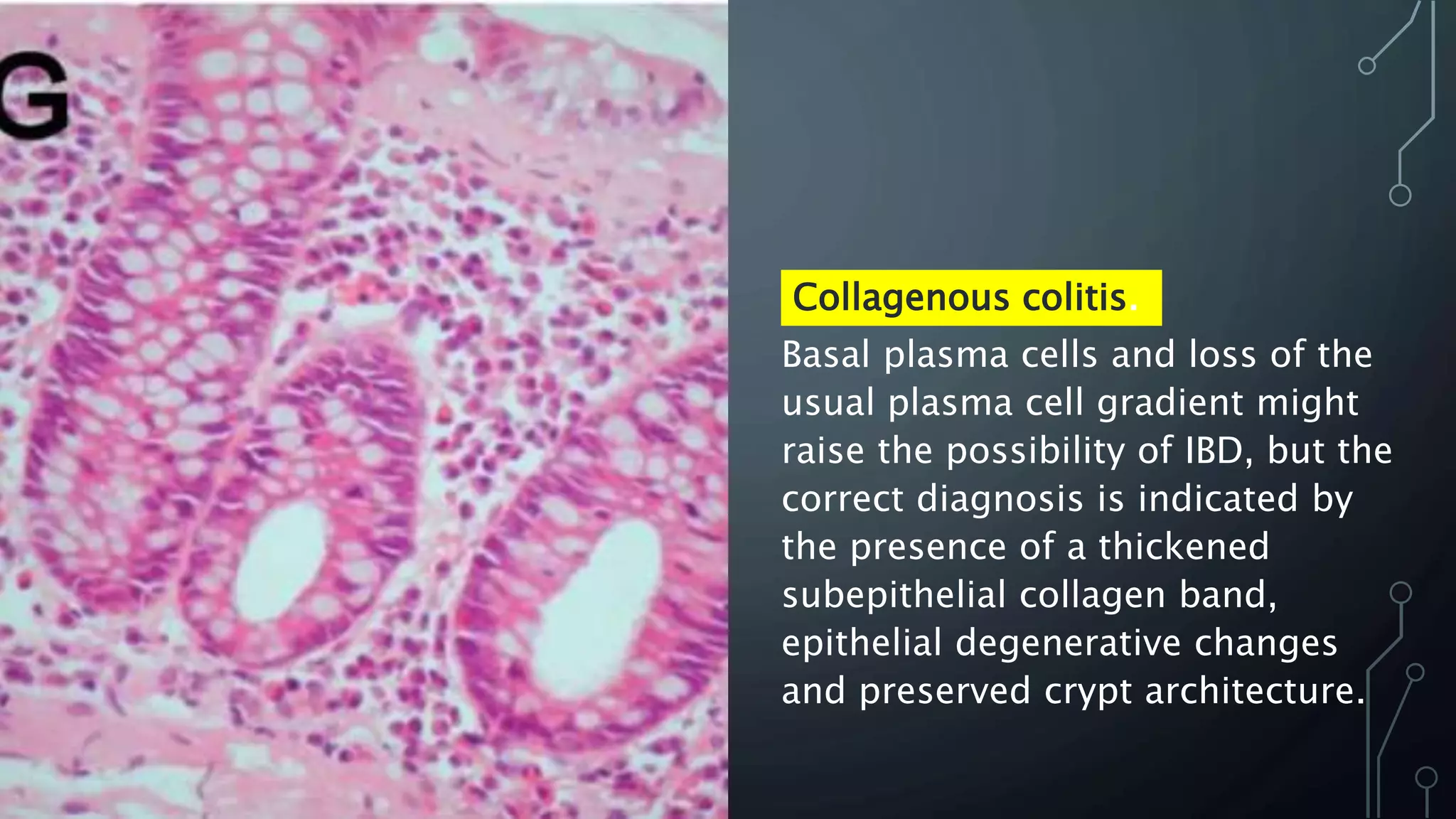
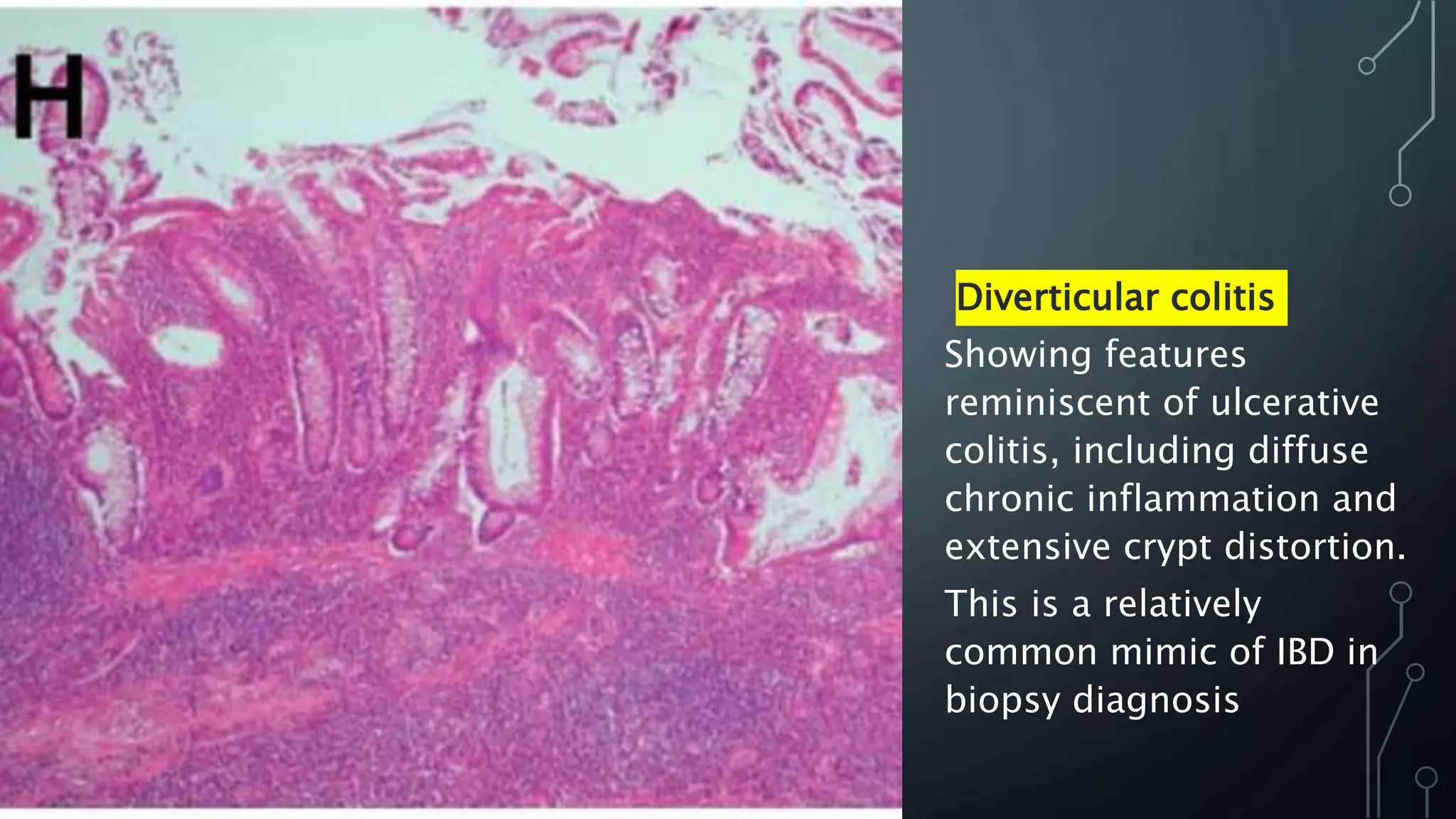
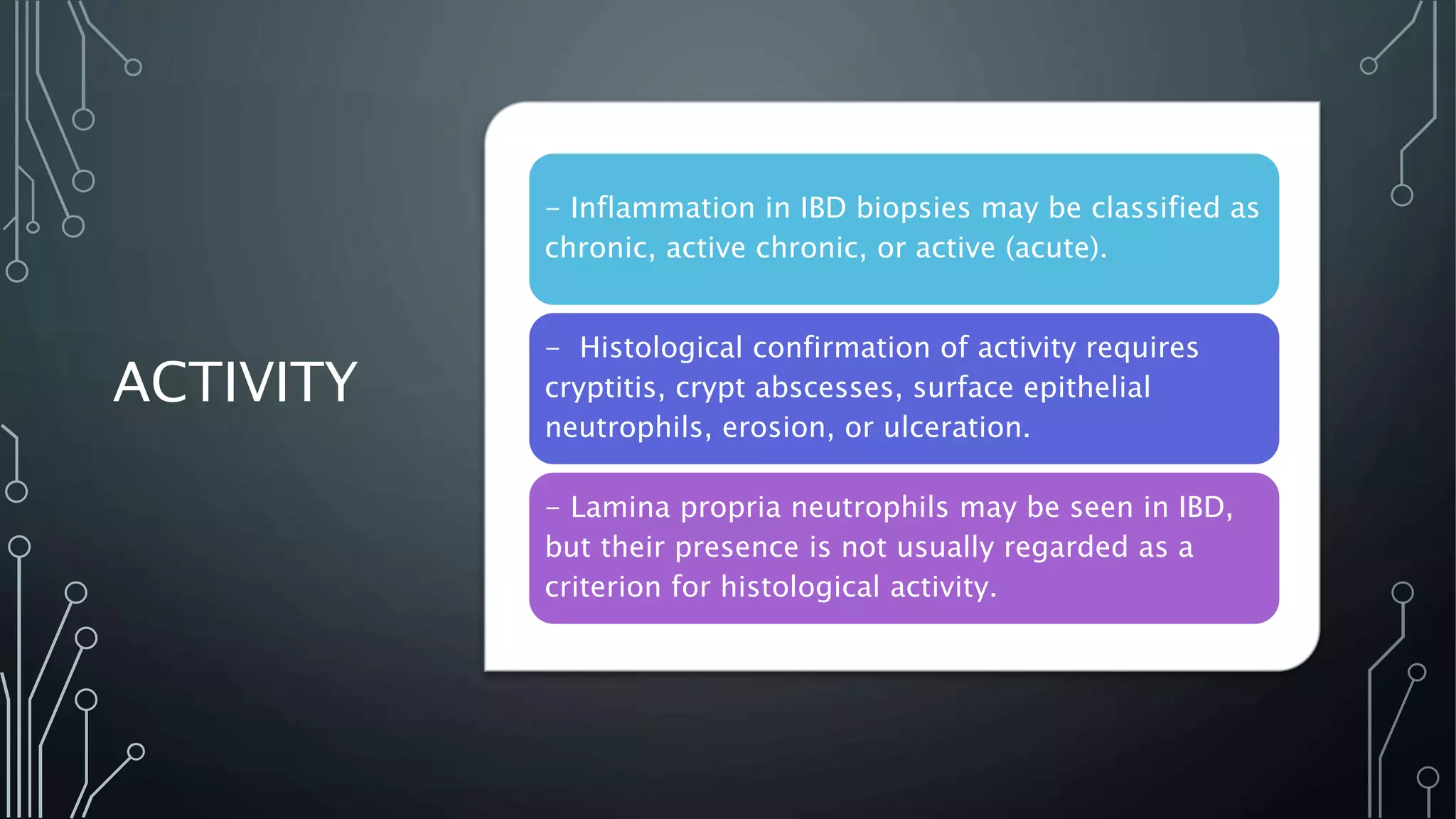
This document provides guidance on histopathological assessment of inflammatory bowel disease (IBD) biopsies. It discusses the clinical considerations and tissue sampling for IBD diagnosis. The key diagnostic categories and features of normal and abnormal mucosa are outlined. Guidance is provided on distinguishing IBD from non-IBD conditions, and distinguishing between ulcerative colitis and Crohn's disease. The effects of time and treatment on histological findings are also covered. The document recommends a standardized reporting scheme called PAID for IBD biopsy reports.