This presentation provides an overview of an oncology-focused immunotherapy company. It discusses the company's lead product, NeuVax, which is an immunotherapy targeting HER2-positive breast cancer in both the adjuvant and metastatic settings. Key information includes:
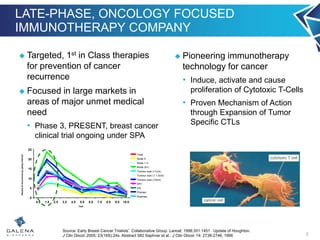
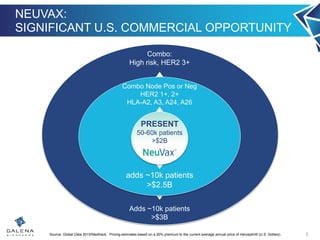
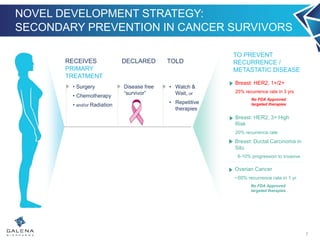
- NeuVax is currently in a Phase 3 clinical trial (PRESENT) in the adjuvant setting for HER2 1+/2+ breast cancer patients. Enrollment is complete for the trial.
- An interim analysis of the PRESENT trial is expected in late Q2 2016 which will evaluate safety and futility based on 70 recurrence events.
- Additional clinical trials are exploring NeuVax in combination with Herceptin and in other HER2-positive cancers like