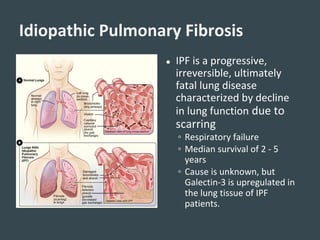
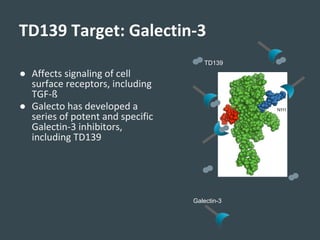
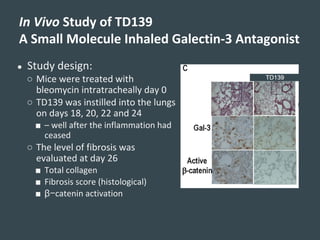
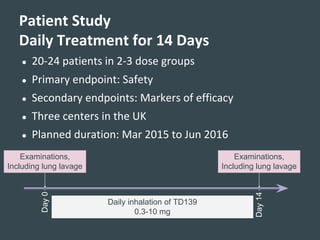
Galecto Biotech is focused on developing treatments for fibrotic diseases by targeting galectins, proteins involved in fibrosis. They have developed TD139, a potent and specific galectin-3 inhibitor, as a treatment for idiopathic pulmonary fibrosis (IPF). Preclinical studies in mice showed TD139 reduced fibrosis when delivered via inhalation after bleomycin-induced lung injury. A first-in-human study of inhaled TD139 found it was well tolerated with no dose-limiting toxicities. Galecto is currently conducting a Phase II clinical trial testing multiple doses of inhaled TD139 in IPF patients to evaluate safety and efficacy markers.