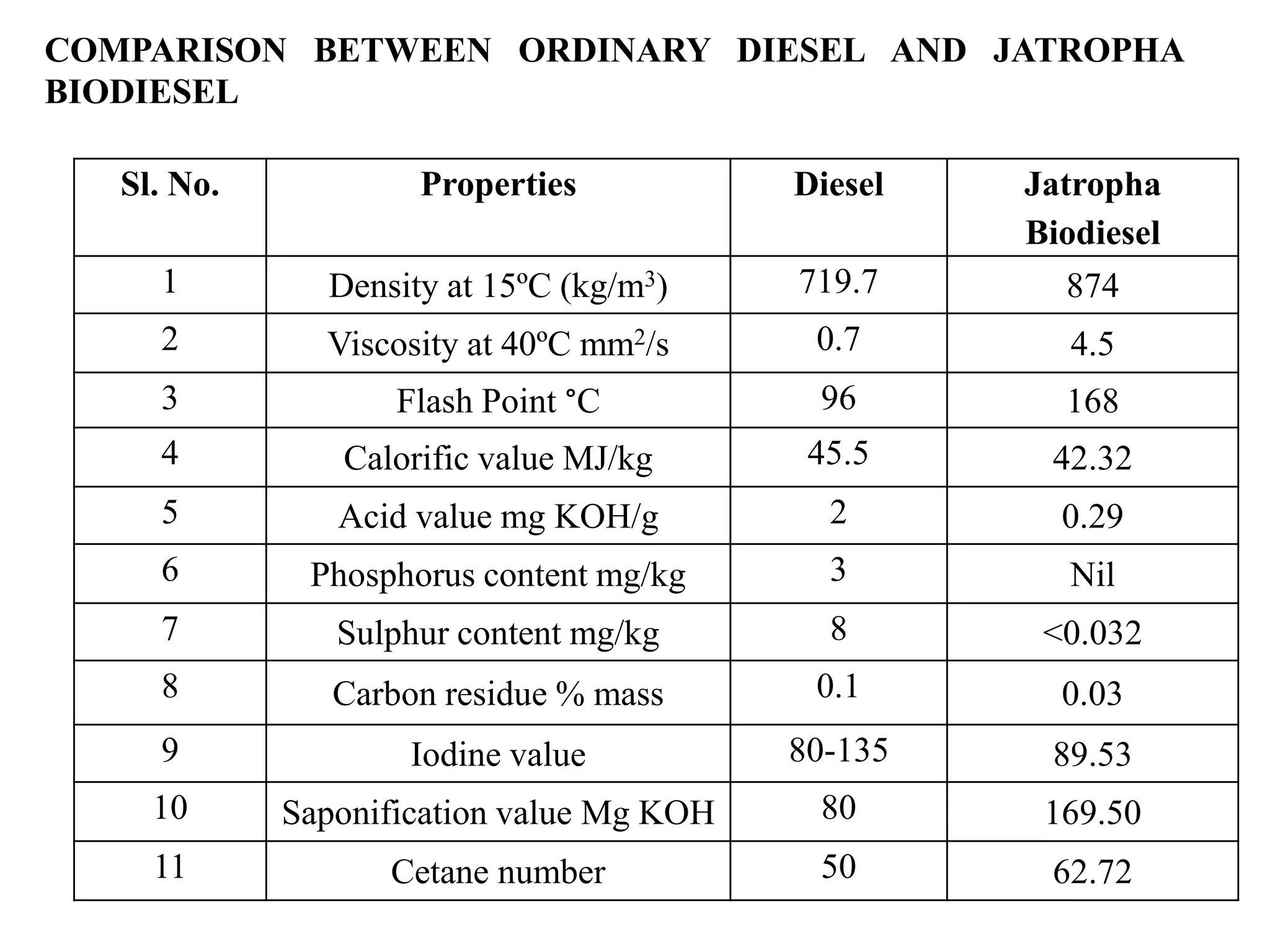
This document summarizes research on producing biodiesel from macroalgal biomass using natural catalysts. It discusses how macroalgal biomass is a suitable feedstock for biodiesel production. The study used Salvinia molesta macroalgae collected from various locations in India. Various pre-treatment methods were tested to extract oil from the biomass most efficiently. Acid-base catalyzed transesterification was performed on the extracted oil to produce biodiesel. Analysis showed the biodiesel met specifications for properties like density, viscosity, and flash point. The biodiesel produced from macroalgal oil has potential as an alternative fuel and offers economic and environmental benefits.